Exploring the molecular origin of blood clot flexibility
How do blood clots maintain that precise balance of stiffness for wound healing and flexibility to go with the flow? Researchers at the University of Pennsylvania School of Medicine and the School of Arts and Sciences have shown that a well-known protein structure acts as a molecular spring, explaining one way that clots may stretch and bend under such physical stresses as blood flow. They report their findings in a Letter in the latest online edition of the Biophysical Journal. This knowledge will inform researchers about clot physiology in such conditions as wound healing, stroke, and cardiovascular disease.
Clots are a three-dimensional network of fibers, made up primarily of the blood protein fibrinogen, which is converted to fibrin during clotting. A blood clot needs to have the right degree of stiffness and plasticity to stem the flow of blood when tissue is damaged, yet be flexible enough so that it does not block blood flow and cause heart attacks and strokes.
In previous research, senior author John W. Weisel, PhD, Professor of Cell and Developmental Biology, measured the elastic properties of individual fibers and found that the fibers, which are long and very thin, bend much more easily than they stretch, suggesting that clots deform in flowing blood or under other stresses, primarily by the bending of their fibers.
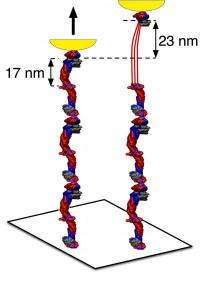
The current research extends those earlier findings to the molecular level, suggesting a way that individual fibers flex - by the unraveling of the three, tightly twisted rod-like regions within fibrinogen molecules, called alpha-helical coiled-coils. The researchers measured this change by pulling engineered strands of fibrinogen molecules using an atomic force microscope. This alpha-helical coiled-coil "spring" is a common motif in protein structure, first identified more than 50 years ago and so its stretchiness may have broader implications in biology and medicine.
By understanding mechanical processes at the molecular level, it may eventually be possible to see how they relate to the mechanical properties of single fibers and a whole clot. This knowledge may enable researchers to make predictions about the function of differently formed fibrin clots in the circulating blood or in a wound. For example, when clots are not stiff enough, problems with bleeding arise, and when clots are too stiff, there may be problems with thrombosis, which results when clots block the flow of blood. First author André Brown, a physics graduate student at Penn, notes that this research is a first step towards understanding the mechanics of the relationship between clot elasticity and disease.
Recent research by other scientists showed that a fibrin fiber could stretch four to five times its original length before snapping. "This is among the most extensible, or stretchy, of polymers that anyone has ever found," says Weisel. "But, how is the stretching happening at a molecular level? We think part of it has to be the unfolding of certain parts of the fibrin molecule, otherwise how can it stretch so much?"
Previous research from senior coauthor Dennis Discher, PhD, Professor in the Physics and Cell & Molecular Biology graduate groups, suggested the possibility that alpha-helical structures in some blood-cell proteins unfold at low levels of mechanical force. But "it wasn't known before that the coiled coil region of the fibrinogen molecule would be the part to unfold under the stress induced by the atomic force microscope," notes Brown.
Once the origins of the mechanical properties of clots are well understood, it may be possible to modulate those properties, note the study authors. "If we can change a certain parameter perhaps we can make a clot that's more or less stiff," explains Weisel. For example, various peptides or proteins, such as antibodies, bind specifically to fibrin, affecting clot structure. The idea would be to use such compounds in people to alter the properties of the clot, so it can be less obstructive and more easily dissolved.
In the future, the researchers will examine other processes at the molecular and fiber levels that may be responsible for the mechanical properties of clots to eventually develop a model that can then be used to predict the effect of changes at one scale on clot properties at other scales. Such a model should be useful for developing prophylactic and therapeutic treatments for many aspects of cardiovascular disease and stroke, suggest the investigators.
Source: University of Pennsylvania School of Medicine