Simulations Show Liquid Water Could Exist on Mars

University of Arkansas researchers have become the first scientists to show that liquid water could exist for considerable times on the surface of Mars.
Image: This Petri dish contains the Mars soil stimulant used in the experiments inside the simulation chamber. Note the layers of ice and dust and the darkening of the dust. Also, water has flowed out onto the surface and in some places lies over dry soil. The mud forms a seal and helps retain water. Photos courtesy of Derek Sears.
Julie Chittenden, a graduate student with the Arkansas Center for Space and Planetary Sciences, and Derek Sears, director of the Space Center and the W.M. Keck Professor of Planetary Sciences, will report their findings in an upcoming issue of the Geophysical Research Letters.
"These experiments will help us understand how water behaves on Mars," Chittenden said.
Researchers have debated whether or not liquid water could exist on the surface of Mars because of the low temperatures and pressures found on the planet. Based on previous experiments and hypotheses, scientists have speculated that pure water on the planet's surface would evaporate from solid to gas, bypassing the liquid phase, at the low pressures found on Mars - 7 millibars as opposed to about 1,013 millibars on Earth. However, the planet's surface sports features like gullies and channels that look as though they might have been created by the movement of liquid. Terrestrial experiments designed to simulate Mars-like conditions have been performed to help answer this question of whether or not liquid water exists on Mars, but until this point they have only been done with pure water at high pressures.
Chittenden and Sears used a planetary environmental chamber in the W.M. Keck Laboratory for Space Simulation to simulate the conditions found on Mars - an atmosphere of carbon dioxide, 7 millibars of pressure and temperatures from zero degrees Celsius to 25 degrees below - and examined the evaporation rates of brine solutions expected to be found on Mars. Most water on Earth contains salts that leech into the water when it comes in contact with soil, and similar processes might be expected to occur in any surface water found on the Red Planet. Salts in the water lower the freezing point of the solution.
The University of Arkansas team placed the salt solutions in the planetary environmental chamber simulating Mars-like conditions, and then measured the evaporation rates at varying temperatures.
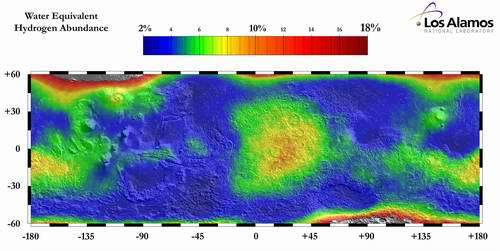
Image: This hydrogen distribution map from Los Alamos National Laboratories shows where hydrogen that may be tied up in water exists on Mars. Photos courtesy of Derek Sears.
"There's a huge decrease in the evaporation rate the colder it gets, more than anyone realized," Chittenden said. With the dissolved sodium and calcium in the water, the freezing point for the brine mixtures drops to 21 degrees below zero Celsius for salt water and 50 degrees below zero for water containing calcium chloride.
Temperatures on Mars vary between 125 degrees below zero Celsius and 28 degrees above at different latitudes and different times of the day. Thus, there is a possibility that liquid water could exist on the planet's surface at different locations and times of day.
"Brine formation could considerably increase the stability of water on Mars by both extending the temperature range over which liquid water is stable to negative-40 degrees Celsius and by decreasing the evaporation rates by two orders of magnitude," the researchers wrote.
Source: University of Arkansas