H. peroxide sensor could aid security
A new family of molecules used to detect hydrogen peroxide and other reactive chemicals in living cells could be a useful addition to anti-terrorist arsenals, says the University of California, Berkeley, chemist who developed these substances last year.
When mixed with a material that contains even trace amounts of hydrogen peroxide, the powdery sensors turn bright yellow or red and light up. "You don't need anything to read the selective reaction other than your eye," said the chemist, Christopher Chang, an assistant professor at UC Berkeley's Department of Chemistry. "You could also use a hand-held black-light lamp to detect the fluorescence."
British authorities say that some two dozen suspects they arrested earlier this month in an alleged plot to blow up as many as 10 jetliners may have been planning to blend liquids on board the crafts to create explosives. Peroxides and acetone top the list of liquids under suspicion.
Chang's sensors, which are non-toxic white powders, could be easily designed and modified to change to any color or to detect any number of peroxides or other chemicals that may be of interest to authorities, he said. "They could check for many things at once," he added. "You'd just have to look for the color."
To make the sensors even easier to use, they could be manufactured in a variety of forms, including paper strips similar to the ones used to measure pH levels, the chemist said. Authorities testing for illicit substances could then simply dip the strip into the suspect liquid or gel and look for a color change.
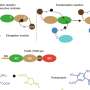
Chang has developed these sensors for use by cell biologists and other researchers working on diseases associated with aging, such as cancer and neurodegenerative diseases. What maladies like these have in common is an unregulated production in cells of compounds that can trigger oxidative damage to tissue and organs. Hydrogen peroxide is a major byproduct of such processes, and its presence is a good indicator that cells have undergone oxidative stress.
The sensors are based on a fluorescent dye called fluorescein used in many biological research applications. They are composed of small molecules that incorporate what Chang calls "chemical cages," molecular fragments he designed to react selectively with hydrogen peroxide. In what is known as a cleavage reaction, hydrogen peroxide strips boronates off of this class of molecules, turning them into their fluorescent counterparts.
Chang's paper describing these substances was published in the Journal of the American Chemical Society in November 2005. Co-authors of the paper are Evan Miller and Aaron Albers at UC Berkeley's Department of Chemistry and Arnd Pralle and Ehud Isacoff at UC Berkeley's Department of Molecular and Cell Biology.
Source: UC Berkeley