Hydrogen ions caught in the act of wandering
Erik T.J. Nibbering of the Max Born Institute for Nonlinear Optics and Short Pulse Spectroscopy (MBI) and colleagues report for the first time experimental evidence of the motions of hydrogen ions (protons, H+) from acids via water to bases. Until now this has only been estimated as a possible reaction mechanism with theoretical calculations. With this study, the international research team provides insight into fundamental processes in nature (acid-base neutralization, proton transmission through water and through biomembranes), that may well become relevant for technological applications, e.g. in fuel cells.
The scientists report on these findings in Science (Vol. 310, pp. 83 – 86) Nibbering’s team consisted of his colleagues from the MBI, Omar F. Mohammed (a Ph. D. student from Egypt) and the theoretician Jens Dreyer, and the group of Ehud Pines at Ben Gurion University of the Negev (Israel).
For a long time, it was not clear how the transfer of protons in aqueous solutions occurs. This is because protons do not move freely in water, but form complexes with water molecules (H2O) through hydrogen bonds. Hydronium (H3O+) is formed, but this ion will not stay alone, because it forms complexes with nearby water molecules in continuously exchanging configurations, e.g. in the form of the so-called Zundel (H5O2+) and Eigen (H9O4+) cations. Erik Nibbering and colleagues succeeded to make snapshots of the proton motions with ultrashort laser flashes. It turned out that hydrogen ions are transmitted from acid to base by water molecules.
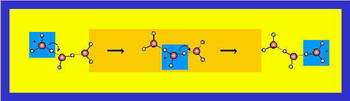
Fig. 1: Grotthuss mechanism for proton conductivity in water. Protons are shown in grey
Hydrogen ions are transmitted very efficiently through water. First theoretical considerations on this were made exactly 200 years ago by the german-baltic scientist Theodor von Grotthuss, and since exactly 100 years scientists use the phrase “Grotthuss mechanism” to indicate the jump-like transmission of protons to neighbouring water molecules (see Fig. 1). “One can use the picture of the improving a dike with sandbags”, says Nibbering. A chain of people will transport the sandbags more efficiently and faster towards the dike than everybody on his own. “You could speak of proton hopping”, explains Nibbering. Only recently, numerous theoretical refinements have become available. Detailed calculations, for example, made clear that proton transmission becomes possible when the surrounding water rearranges at particular points in time to enable the Zundel-cation and at other times the Eigen-cation configuration.
Furthermore, theoreticians have derived that the exchange of protons between acids and bases in aqueous solution should occur in a similar fashion. Now, the recent report in Science confirms the hopping model (Fig. 2).
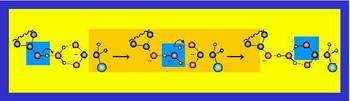
Fig. 2: Here the proton transfer reaction proceeds from an organic acid towards a carboxylic base via an intermediate hydronium stage with an Eigen-cation configuration.
The experimental study has become possible by a technique, that enables the determination of the reaction progress in time steps of 150 femtoseconds. This is extremely fast. For comparison: A laser beam will reach the moon in one second. In 100 femtoseconds on the other hand a laser beam will only have reached a distance equivalent to the diameter of a human hair. The scientists have used in their experiments an aqueous acid-base mixture, with which they already have been performing proton transfer studies since two years. “Two years ago, we were not able to observe the intermediate steps. We could only see the beginning and the end of the proton transfer reaction”, says Nibbering. By a change of the components of the acid-base mixture the reaction has been slowed down so that now the sequential proton hopping via water molecules can be recorded.
Source: Forschungsverbund Berlin e.V. (FVB)


















