Captive Electrons
Let’s imagine a really small country - say, Liechtenstein - won the World Cup of soccer. It would be quite a sensation. But how’s this for a sensation: what if the soccer ball, in the middle of the match, suddenly decided to hang suspended in mid-air? (Well if something that "unnatural" happened, probably people wouldn’t care that much about the outcome of the World Cup anymore.) What if the ball only started flying again after a player went up and kicked it. This all seems pretty much impossible for a soccer game, but now something similar, and very, very improbable, has happened in the world of quantum physics.
Scientists at the Max Planck Institute of Microstructure Physics in Halle, Germany, have "kicked" the electrons in a metal plate in a vacuum with a laser light in such a way that they populated states above the vacuum level. For some of them that means they escaped the metal’s lattice, but just sort of hung suspended over the surface of the metal - even though the scientists would have expected them to fly into the vacuum. These electrons ended up in a state that scientists have only imagined, never thought to be really possible. (Physical Review Letters, March 3, 2006)
We wouldn’t have computer chips, lasers, and neon lights, if it weren’t for research into the electronic characteristics of natural and artificial materials. Scientists try to determine where electrons are located in these substances - or, as the scientists might put it, with which energy the electrons whip around atomic nuclei - and how to give these electrons an energetic "kick". For this kind of work in solids, researchers use photoelectron spectroscopy. Their methods are based on the work on the photoelectric effect which won Albert Einstein the Nobel Prize in 1921.
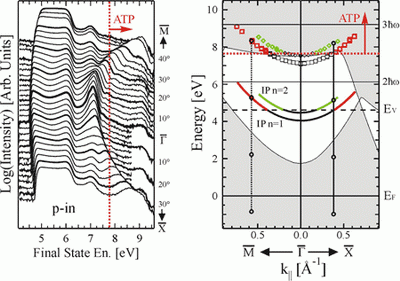
There are still many secrets hidden even in well-known metals like copper, and much beyond the simple theory of photoelectric effect developed by Einstein. Francesco Bisio and Miroslav Nývlt, guest researchers at the Max Planck Institute of Microstructure Physics in Halle, Germany, in collaboration with Hrvoje Petek from the University of Pittsburgh, have now taken copper plates and observed electrons in states which lie above the vacuum level, which is the minimum energy that an electron needs to be freed from the metal’s lattice. The electrons do not, however, escape; rather, they stay held near the metal’s surface.
Until now these states were thought to exist only as virtual states, which means that the electrons were thought to spend no time in these states, but researchers showed this not to be their case.
Jürgen Kirschner, Director at the Max Planck Institute and head of Bisio and Nývlt’s department, says, "my two colleagues have now shown that these conditions are real, not virtual - even if they just exist for a very short time." It is thanks to a quirk in quantum theory that electrons can take on such unusual states. The probability that electrons are excited in the way Bisio and Nývlt achieved is in fact virtually nil, and depends on how many photons they shoot at the electrons - how often, that is, they get "kicked". Comparatively, it is much more likely that Liechtenstein really won the World Cup.
Bisio and Nývlt used laser light at an energy which Einstein would have said was not high enough to knock a photon out of copper. But the electrons lassoed in a number of photons at the same time, collecting together the energy necessary to escape the metal lattice. In the theory, Einstein proposed that only the colour (the energy) of the light, and not its intensity, determines whether electrons can be ejected from a metal. But Einstein never knew what a laser was. Lasers deliver light pulses so intense, that they can induce phenomena which physicists label "higher order" or "non-linear". Scientist have been using lasers in this way for some time already, but Max-Planck-Physicists, for the first time, have now carried this procedure to the fourth grade of non-linearity.
It is an extremely improbable phenomenon. Bisio and Nývlt were only able to observe it because they used very intense pulses of light. Kirschner calls it "a curious thought: non-linear quantum effects make phenomena possible which classical physics would call linear - and which we have thought, since the time of Einstein and Planck, is a physical impossibility."
Bisio and Nývlt had another trick up their sleeve, besides the high intensity light. They did not just gather all electrons emitted from the copper plate. Rather, they only collected electrons that were emitted at particular angles from the surface. In these conditions they could see electrons that were not just moving away from the metal’s surface, but mostly parallel to it. The electrons moved around quite a bit - but only on a single plane above the copper plate. It was as if a soccer player kicked a ball - and it just hovered, rather than flew out of the stadium.
Citation: Francesco Bisio, Miroslav Nývlt, Jiri Franta, Hrvoje Petek and Jürgen Kirschner, Mechanisms of High-Order Perturbative Photoemission from Cu(001), Physical Review Letters, 3 February 2006
Source: Max Planck Institute















