Crystal tears
"For a tear is an intellectual thing", said William Blake in Jerusalem, and Peter Petrov of the University of Exeter and colleagues have shown how right he was. They have found that tears, far from being merely salty water as in romantic tradition, are extremely complex fluids whose surfaces are highly structured in a manner reminiscent of cell membranes.
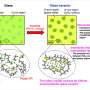
The tear film that covers and moistens our eyes has to serve several functions: in particular, it must keep debris and microorganisms out, while holding the water in and preventing it from evaporating. That is why this liquid film has a protective coating just one or two molecules thick, rather like the surface of a soap bubble. Petrov and colleagues have investigated how this coating, made up of a mixture of many different biological molecules, is arranged, so that they can gain some idea of how it fulfils its role.
Some of the molecular components of a tear film's 'skin' are indeed soap-like molecules: called lipids, they are similar to the key constituents of cell membranes, and have a water-soluble 'head' and an insoluble 'tail'. At the surface of water, these molecules tend to sit in layers one molecule thick, with their water-loving heads immersed and their insoluble tails poking up out of the water. But some other constituents of tear films are wholly 'water-fearing' (hydrophobic) - they will dissolve readily in fats, but hardly at all in water. On their own, such molecules tend to clump together in droplets on the water surface, like droplets of oil or fat.
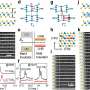
How does this mixture organize itself in a tear film? Petrov and colleagues have attempted to answer this question by bouncing X-rays off the surface of both natural tear films (taken from cows) and artificial analogues composed of a comparable mixture of lipids and oily compounds. These studies showed that, in both the real and the synthetic films, the molecules seem to line up at the water surface in regular, orderly arrays, rather like two-dimensional crystals.
When the researchers added fluorescent molecules to synthetic tear films containing just the lipid components, they saw that the lipids separated into two different states: a relatively disorderly state, like a two-dimensional liquid, interspersed with blobs of a more closely packed, crystal-like state. These lipid crystals grew into remarkable patterns shaped rather like flower heads.
When the fat-like components were added to these artificial films, they seemed to form a separate later on top of the lipids, which enabled them to remain out of the water. Petrov and colleagues think that this arrangement enables the tear film to keep a relatively constant structure even when it is severely squeezed and stretched, as is likely to happen for example when we blink: squeeze the film and the lipid crystals grow a bit bigger; stretch it out and they become smaller again.
Petrov and colleagues will describe their findings at the Condensed Matter and Materials Physics conference in Exeter, organized by the Institute of Physics, on Thursday 20 April.
Source: Institute of Physics