Evidence Suggests a Possible New Phase of Ice
Researchers at the National Synchrotron Radiation Research Center (NSRRC) announced that they have obtained spectroscopic evidence suggesting that a possible new phase of ice may exist at temperatures between 4 K to 50 K, under high pressure.
This discovery was made while a team of researchers led by Dr. Yong Q. Cai were investigating the ordering of hydrogen bonds in H2O under high pressure and at extremely low temperatures. The team confirmed the density functional theory (DFT) calculations under these conditions which correctly account for the pre-edge feature of ice. However, quite unexpectedly they also obtained data indicating substantial spectral changes from ice IX, suggesting a significant change of the H2O framework in this P-T regime. In short, the exciting prospect of the formation of a possible new ice phase.
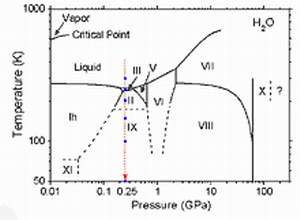
Image: Phase diagram of H2O
It is known that chemical reactions and physical phenomena, which do not occur at ambient pressure, can occur under high pressures and/or extreme temperatures. For instance, acetylene polymerizes without a catalyst, and iodanil shows superconductivity under high pressure. Using inelastic X-ray scattering from NSRRC synchrotron beamline SP12XU at the SPring-8 facility in Japan, at 9884.7 eV with a total energy resolution of 305 and 175 meV, Dr. Cai and his team studied the structure of oxygen in liquid water and ices II, III, and IX near the so-called K-edge state at 0.25 GPa and temperatures down to 4 K. They found that the pre-edge intensity depends sensitively on the number of uncoordinated donor hydrogen bonds and can be used to a large extent as a fingerprint for the degree of hydrogen bond ordering of the H2O framework.
Previously, scientists had conducted experiments under even higher pressures, e.g. 170 GPa with temperatures ranging from 200 – 400 K in order to investigate the change of hydrogen bonds in H2O. Yet, by combining pressure with extremely low temperatures, Dr. Cai’s team was able to explore the water molecules for the first time in more detail. The study also confirms the information on the bonding structure of the water molecules in liquid form on the subfemto-second time scale of the X-ray absorption and emission processes. This discovery gave researchers positive indication on their current approach and direction on finding the possible new form of ice. Further study is in progress.
One of the factors leading to the success of this research effort was the technique underlying the experiment. Since the energy of a soft X-ray can provide only submicron penetration depth on the surface, applying soft X-rays to probe oxygen near the K-edge may introduce uncertainties due to surface sensitivity. Another challenge was the fact that the energy levels of soft X-rays are usually incompatible with the high-pressure vessel, the Diamond Anvil Cell (DAC), used in Dr. Cai’s experiment. The solution that delivered the success for Dr. Cai’s team was to adopt an alternative technique, called ‘X-ray Raman Scattering (XRS)’, with the use of hard X-rays (~10 keV) to induce core-level excitations for better penetration.
The study of the electronic structure of hydrogen bonds in various phases of H2O holds the key to the discovery of the bonding framework and the bonding changes among ionic and covalent hydrogen. This can be instrumental in learning the physical and chemical properties of organic and biological systems, particularly at high pressures, low temperatures, or both. Hydrogen bonding also plays an important role in determining the three-dimensional structures adopted by proteins and nucleic acids. The double helical structure of DNA, for example, is due largely to hydrogen bonding between the base pairs, which link one complementary strand to the other and enable replication.
Although a fundamental and complete understanding of the hydrogen bond in ice is still an open question, advances in synchrotron X-rays are making a positive impact in this direction. As well as being more accessible, modern synchrotron X-rays incorporate instruments with higher levels of accuracy, and can utilize combinations of X-ray spectroscopy, photoelectron spectroscopy and DFT calculations. This enables researchers to observe important rehybridization characteristics inside water molecules due to hydrogen bonding more closely than ever before.
Source: National Synchrotron Radiation Research Center















