CardioMEMS Moves Closer to Commercializing Innovative Sensors for Heart Patients
CardioMEMS, a member of Georgia Tech’s Advanced Technology Development Center (ATDC), is pioneering a new breed of testing devices to monitor heart patients. Combining wireless communications technology with microelectromechanical systems (MEMS) fabrication, CardioMEMS’ products can provide doctors with more information while making testing less invasive for patients.
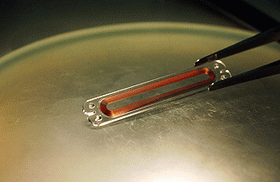
Image: Close-up image of a CardioMEMS pressure sensor. The device is implanted along with a stent grant to wirelessly measure pressure. The device is currently undergoing clinical trials. Georgia Tech Photo: Gary Meek
In June, the U.S. Food & Drug Administration (FDA) approved CardioMEMS’ investigational device exemption (IDE), which enabled the company to begin clinical trials in the United States for its EndoSensor™.
The EndoSensor measures blood pressure in people who have an abdominal aortic aneurysm, a weakening in the lower aorta. This condition ranks as the 13th leading cause of death in the United States. If the aneurysm ruptures, a person can bleed to death within minutes.
Doctors can treat the aneurysm with a stent graft, a slender fabric tube placed inside the bulging artery to brace it and relieve pressure by creating a channel for blood flow. Still, the stent can fail, resulting in leakage of blood into the aneurysm, which can cause the aneurysm to burst. For this reason, lifetime monitoring is required.
Safer, easier testing
Up to now, doctors have relied on CT scans for testing, but CT scans have limitations. “One problem is that CT scans only show the size of the aneurysm,” explains David Stern, CardioMEMS’ chief executive. “Yet pressure, which is what our device monitors, is the most important measurement.”
CT scans are also time-consuming and expensive, Stern adds. And for patients who require lifetime monitoring, there’s a safety issue due to repeated exposure to radiation and contrast dyes that are toxic to kidneys.
CardioMEMS’ biocompatible sensor, which is implanted along with the stent, monitors the stent more effectively than CT scans. It’s also cheaper and more convenient. During checkups, patients don’t even need to remove their clothes. The physician merely waves an electronic wand in front of the patient’s chest. Radio-frequency waves activate the EndoSensor, which takes pressure measurements and then relays the information to an external receiver and monitor.
CardioMEMS conducted its first U.S. implants at the Cleveland Clinic in July. By the end of December, approximately 100 patients in four countries (the United States, Canada, Argentina and Brazil) had received sensors. CardioMEMS will submit resulting trial data to the FDA early this year, and Stern hopes to receive permission to start selling the EndoSensor by mid-2005.
“Our trials show the EndoSensor is safe and producing good data,” reports Stern. “Doctors are enthusiastic because the sensor is very easy to use even though it’s complex technology.”
Early warning system
Separately, CardioMEMS has also been advancing its HeartSensor, a wireless device that measures intracardiac pressure in patients with congestive heart failure.
Similar to the EndoSensor, the HeartSensor is inserted through a catheter in a non-surgical procedure. Patients receive monitoring electronics to take home, which are used to conduct daily pressure readings. Then that data is transferred over a phone line to their physician.
“Our HeartSensor enables doctors to monitor patients more closely and adjust medications as they see the disease progressing,” Stern explains. “Because the sensor detects a change in the body before any external symptoms are manifested, it serves as an early warning system and prevents patients from ending up in the hospital.”
CardioMEMS began initial animal studies last fall and has successfully implanted the heart monitor in pigs. This year Stern hopes to begin uman trials both in and outside of the United States.
Other events accelerating CardioMEMS’ commercialization efforts include:
-- External manufacturing. Early prototypes were manufactured at Georgia Tech, but CardioMEMS has signed an agreement with an established MEMS foundry to produce the EndoSensor. The company has already demonstrated the sensors can be produced in large quantities at an affordable cost.
-- FCC license. The Federal Communications Commission has granted CardioMEMS a license to operate its wireless sensors in its required frequency range.
-- Patent protection. The U.S. Patent and Trademark Office has allowed claims on several patents that CardioMEMS filed earlier.
Launched in 2001, CardioMEMS was co-founded by Dr. Jay Yadav, a cardiologist and director at the Cleveland Clinic Foundation, and Mark Allen, a professor in Georgia Tech’s School of Electrical and Computer Engineering and director of the school’s MEMS research group.
Yadav was interested in Allen’s use of MEMS technology for microsensors that could measure pressure in turbine engines. Although Allen had designed the sensors specifically for military drone aircraft, he and Yadav believed that they could adapt the technology to monitor heart and blood pressure in humans.
MEMS technology uses micro-machining fabrication, which was originally developed for the integrated circuit industry to build electrical and mechanical structures at the micron scale (one-millionth of a meter). “MEMS is an attractive platform for medical devices because mechanical, sensory and computational functions can be placed on a single chip,” Sterns explains. “It also has a low cost of manufacturing and is capable of extreme miniaturization.”
Admitted to ATDC in 2001, CardioMEMS has grown to 30 employees. “ATDC has given us access to a range of personnel and facilities that have been instrumental to our success,” Stern says, noting that one-third of the company’s employees are either Georgia Tech graduates or students working part-time.
CardioMEMS has already raised $16.5 million in funding, which includes a $14 million infusion in Nov. 2003 – a coup in light of the difficult investor environment. “We’re in a good cash position right now, but we’ll be looking at raising more money to fund human trials of our HeartSensor,” Stern says.
Source: Georgia Institute of Technology
















