Hydrated Electrons Can Take More Than One Guise
Scientists with the U.S. Department of Energy's Lawrence Berkeley National Laboratory appear to have settled a long-standing scientific question about water clusters – aggregates of water molecules that feature unique properties, somewhere between that of liquid water and steam. Experiments led by Daniel Neumark, director of Berkeley Lab's Chemical Sciences Division, have identified two distinct forms of negatively charged water clusters, thereby providing new insight into the fundamentally important interaction between electrons and water.
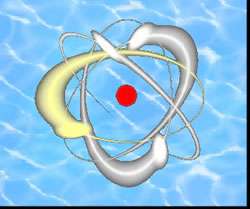
Image: In the traditional view, a hydrated electron (shown in red) is confined within a small void created by a surrounding cluster of water molecules. Berkeley Lab researchers have found an alternative structure in which the hydrated electron may be bound to the surface of the cluster
"We have confirmed the presence of two isomers of water cluster anions: internally solvated structures, in which a hydrated electron is localized within the cluster; and surface state structures, in which the hydrated electron is bound to the surface of the cluster," Neumark says. "The internally solvated structures are the ones whose properties should approach those of the bulk liquid hydrated electron as the cluster size is increased."
Neumark is the principal author of a paper published in Science Express, the on-line version of the journal Science, entitled Observation of Large Water Cluster Anions with Surface-bound Excess Electrons. The other authors were Jan Verlet, Arthur Bragg, Aster Kammrath of the University of California at Berkeley, where Neumark is a professor of chemistry, plus Ori Cheshnovsky, of the Tel-Aviv University in Israel. This and a companion paper published by Neumark's group earlier this year were listed as one of the "runner-up" Breakthroughs of 2004 by Science.
Hydrated electrons form when an excess of electrons are injected into liquid water. Despite being the focus of numerous studies since their discovery in 1962, there remains much to be learned about hydrated electrons. What is known is that their presence enhances the reactivity of water molecules with other molecules in a number of important chemical, physical and biological processes.
The long-held belief has been that an individual hydrated electron is confined within a small void created by a surrounding cluster of water molecules. Clusters, which may consist of as few as three or as many as 20,000 individual atoms in size, are too large to be thought of as a molecule but too small to be classified as a bulk-phases liquid or a solid. Because of their in-between size, they often make excellent subjects for learning more about the physical and chemical properties of bulk phase materials.
"In our lab, we carry out experiments that help us understand how phenomena associated with macroscopic materials manifest themselves in finite clusters," Neumark says.
One of the key questions his group has been addressing concerns the relationship of a hydrated electron to the size of the water cluster surrounding it. How large can the cluster be for the effects of the hydrated electron to mimic the effects in bulk phase liquid, and do those effects change as the cluster grows in size? Previous studies indicated the presence of hydrated electrons in water clusters but found conflicting results as to their effect.
"The problem was those earlier studies couldn't determine that the clusters could have either an internally solvated or a surface hydrated electron structure," Neumark says. "Our experiment pretty much settles this issue."
Neumark and his colleagues created clusters of water anions by passing argon gas over molecules of water and heavy water at temperatures of 20 degrees Celsius, introducing the gas mixture into vacuum, and generating negatively charged clusters through the interaction of the gas mixture with low energy electrons. The clusters were then studied using a combination of femtosecond laser light and time-resolved photoelectron imaging. This application of time-resolved techniques to gas phase processes occurring on a femtosecond time scale has been one of the most important developments in chemical dynamics during the last ten years and has yielded valuable information on the photo dissociation and reaction dynamics of molecules and clusters
In this latest effort, Neumark and his colleagues were able to characterize an entirely new class of cluster anions with vertical binding energies (the energy required to remove an electron from its orbit) that were significantly lower than any previously recorded.
"The data are consistent with a hydrated electron structure in which the excess electron is bound to the surface of the cluster," Neumark says. "This result implies that previously observed water cluster anions, with higher vertical binding energies, were indeed from internally solvated electrons and are therefore structurally similar to a bulk hydrated electron."
From their findings, Neumark and his colleagues conclude that an anion water cluster needs to consist of at least 11-25 molecules in order to be able to have distinguishable internally solvated or surface state structures. They also found that they could create conditions that would favor the formation of one structure over the other.
Says Neumark, "By operating our ion source so that we produced colder clusters, we were able to favor the formation of surface state over the internally solvated structure," says Neumark. "That was somewhat surprising since the internal structures tend to be more stable. For the surface structure, we're basically attaching electrons to ice nanocrystals."
Source: Berkeley Lab



















