Glass gives up secrets under pressure
Glass is a mysterious material, but when researchers apply pressure, it reveals secrets.
Using a variety of techniques, researchers at Argonne National Laboratory saw for the first time ever, the atomic structure of a dense, purely octahedral glass that has eluded scientists for decades. They also witnessed a continuous structural change in the glass, disproving the theory that tetrahedral glasses go through a distinct transition between low- and high-density phases.
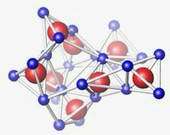
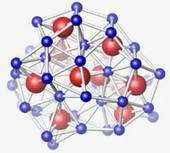
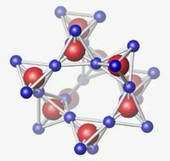
The images show how the structure of germanium-dioxide glass changes as it is subjected to increasing greater pressure. The structural units are about 1 nanometer, or one-billionth of a meter, across. The top image shows the material's basic tetrahedral structure at normal pressures. In the second image, the tetrahedral structure begins to collapse when pressures reach about 50,000 times atmospheric pressure. In the bottom image, at 60,000 to 100,000 times atmospheric pressure, the structure becomes octahedral.
“Little is known about the structure of glass under pressure” said materials scientist Chris Benmore, “even though it is quite important. We put it in our cars and homes, and use it in many industrial applications, but how does the atomic structure react to extreme pressures?”
Benmore is a researcher in Argonne's Intense Pulsed Neutron Source Division (IPNS). This division operates the IPNS, which provides neutrons for condensed matter physics, the study of atomic arrangements and motions in liquids and solids. IPNS is open to researchers from industry, academia and other national laboratories.
Glass is difficult to study because it is disordered and lacks a periodic crystal structure. Also, it needs to be studied under pressure, because, as Benmore said, “the glass structure pings right back when the pressure is lessened.” Researchers designed original pressure cell geometries for the research.
To witness the glass' transition under pressure, Benmore and colleagues used a combination of tools:
-- Neutron diffraction studies at Argonne's IPNS
-- X-ray diffraction at Argonne's Advanced Photon Source, and
-- Molecular dynamic simulations.
Scientist Chris Tulk from Oak Ridge National Laboratory created novel pressure cells for both instruments in conjunction with other pressure-cell experts from the Carnegie Institution.
“Silica is the most important and most widely used glass,” said Benmore, “but we studied the softer germania (Ge) because it is a structural analog to silica and transforms to the octahedral form at much lower pressures than silica. Germania also provides a greater contrast in the neutron and the X-ray studies, so the details appear more clearly.”
At ambient pressure, Ge has an archetypal tetrahedral network glass structure. Four oxygen atoms enclose a germanium atom and share corners to create cages that are only a nanometer across.
Neutron revelations
The researchers began their experiments at the IPNS. Neutrons reveal structural and dynamic properties of materials, and they are sensitive to lighter elements such as oxygen.
Two piston anvils inside the IPNS's Glass, Liquid, and Amorphous Materials Diffractometer squeezed a 100-cubic millimeter sample of germania dioxide (GeO2) to pressures up to five gigapascals, or 50,000 times atmospheric pressure.
IPNS revealed the mechanism of how GeO2's tetrahedra collapse under pressure. Oxygen atoms were seen being squeezed into the sides of neighboring tetrahedra as the cages collapsed and the glass density increased.
In contrast to the IPNS, the APS reveals germanium atoms more clearly and can test smaller samples, which allows studies at higher-pressures. As a 1 cubic millimeter GeO2 sample was pushed from 60,000 to 100,000 times ambient pressure, researchers witnessed the tetrahedral cages collapsing and an average of five oxygen atoms organizing around the germanium atom before the octahedral glass was formed. This average coordination number of five still did not clearly resolve the question of whether this phase change in germania is continuous or discontinuous.
Researchers thought they may have seen a gradual mixture of five- and then six-fold germanium atoms in the structure as the pressure increased, but the result was still unclear. So they called on their colleagues at the National Research Council of Canada to perform molecular dynamic simulations in which a computer calculates molecular structure and behavior from first principles. “The simulations agreed with our data and revealed a germanate anomaly, that allows a distorted five-fold coordination of germanium to exist over a limited pressure range,” Benmore explained. “This provided evidence that germania glass transforms continuously, which disagrees with the popular two-state model.”
As researchers pressurized a GeO2 sample to 150,000 times ambient pressure, they witnessed a dense, disordered octahedral – eight-sided – structure inside glass for the first time. The angles of the internal structures were not the 90 and 180 degrees of a perfect octahedron; instead, the angles were near 90 and 165 degrees.
“We'll continue to study this dense glass since it has never before been characterized,” said Benmore. “It is a challenge because of the pressures needed. Also, some glass scientists thought the glass would immediately crystallize if it became octahedral.”
This research, which has appeared in Physical Review of Letters, Vol. 93, No. 11, was highlighted in the Editors' Choice in the October 1, 2004 issue of Science.
This is not the first time the team of Benmore and Tulk has shown that the two-state polyamorphic theories have been wrong. In 2003, Benmore, Tulk and colleagues discovered new metastable ice forms when studying ice under pressure. These new forms appear to contradict the widely held belief that the phase change in amorphous ice is discontinuous.
Source: Argonne National Laboratory (by Evelyn Brown)















