Researchers construct a device that mimics one of nature's key transport machines
(PhysOrg.com) -- To help protect its genes, a cell is highly selective about what it allows to move in and out of its nucleus. Yet that choosiness is regulated by just a thin barrier, perforated with tiny transport machines called nuclear pore complexes: protein-coated holes surrounded by flimsy, unfolded protein strands. Now, by building an artificial mimic of this membrane barrier and its pores, scientists have discovered a key to its selectivity and, in the process, have found a practical tool for drug development.
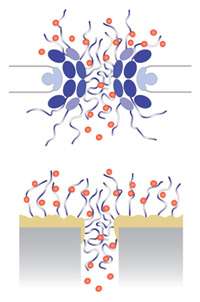
More than 450 proteins make up a single nuclear pore complex. A year ago, Rockefeller University’s Michael P. Rout and Brian T. Chait, along with their colleague Andrej Sali at the University of California, San Francisco, published a detailed structure of the nuclear pore complex, showing for the first time how all of those proteins fit together. Their study showed that, at its simplest, the pore’s organization consists of an anemone-like configuration, with folded proteins forming the hole itself and unfolded, tentacle-like proteins called FG-nucleoporins around the opening.
Now, in their latest study, the researchers are looking at the functionality of the complex at its most basic configuration: a membrane pockmarked with FG-nucleoporin-coated holes. “We wondered whether we could create a simple artificial mimic, made of just a tiny hole and some of these tentacle proteins,” Chait says. “So we built one to see if it really works.”
To do so, postdoctoral associate Tijana Jovanovic-Talisman started with simple polycarbonate membranes strewn with little holes and coated with a thin layer of gold, and then attached a type of FG-nucleoporin to the membrane. Using confocal microscopy, she tested how efficiently proteins crossed this artificial membrane. Then she added transport factors, which bind to the FG-nucleoporins and selectively ferry cargo across the membrane barrier. The researchers saw that transport factors crossed the artificial membrane much faster than proteins alone, just as occurs in the natural nuclear pore complex. Without the transport factors, that selectivity largely disappeared. The research gave the scientists and their collaborators at Los Alamos National Laboratory and the University of Münster in Germany a new perspective on the cell’s nuclear transport mechanism. “We’re beginning to think of the transport factors in a different way,” Jovanovic-Talisman says, “as if they’re a mobile part of the nuclear pore complex machine.”
The results, published online in Nature, do more than explain the minimum needs for the nuclear pore complex’s exquisite selectivity. The artificial pore could be used to test the importance of pore shape and size. And it has potential biomedical applications, too. Because it can separate particular proteins out of very complex mixtures, the device could have enormous implications for biopharmaceuticals. “It’s a device that mimics what nature does and has some beautiful properties, in that it decides what passes through a hole in a very complex mixture,” Chait says.
The team is now working toward making the synthetic pore as selective and efficient as the natural one. “Our machine doesn’t work as well as the nuclear pore complex. We’ve had only three years, while nature’s had billions of years to do this,” Chait says. “We’ve got lots of work to do.”
Paper: Nature online: December 21, 2008 (www.nature.com/nature/journal/ … abs/nature07600.html)
Provided by Rockefeller University