Scientists Present 'Moving' Theory Behind Bacterial Decision-Making
(PhysOrg.com) -- Biochemists at North Carolina State University have answered a fundamental question of how important bacterial proteins make life-and-death decisions that allow them to function, a finding that could provide a new target for drugs to disrupt bacterial decision-making processes and related diseases.
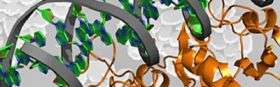
In a study published this month in the journal Structure, the NC State scientists show for the first time that the specific movements of these important bacterial proteins, called transition-state regulators, guide how the proteins bind with DNA and thus control a variety of functions. These rare proteins are like army generals sizing up a battlefield; while they all look the same and have the same rank, their highly specialized "wiggles" allow them to figure out how to bind to different parts of DNA, triggering defense capabilities, for example, or commands to set up camp and chow down.
"For the first time, we've shown that proteins with identical shapes have different movements, and these movements allow proteins to select proper DNA targets that lead to tens or hundreds of processes," says Dr. John Cavanagh, William Neal Reynolds Distinguished Professor of Molecular and Structural Biochemistry at NC State and the corresponding author of the paper. "Motion is really important. If the proteins didn't move, they wouldn't be able to bind to DNA and therefore to function."
Cavanagh and NC State senior biochemistry researcher Dr. Benjamin Bobay, a paper co-author, say that the findings present a new way of thinking about stopping bacteria. If a drug or antibiotic can stymie the motion of the transition-state regulators, the thinking goes, bacteria won't be able to figure out where to bind to DNA, effectively shutting the bacteria down. Killing a general, therefore, would stop the infantry from taking the battlefield.
Besides the fundamental knowledge about bacterial protein movement and DNA binding, the Structure paper also sheds light on the specific bacterial protein responsible for producing anthrax toxins.
One of the transition-state regulators studied by the NC State biochemists, called AbrB, helps control the production of the three toxins in anthrax: lethal factor, edema factor and protective antigen. Production of all three of these toxins is necessary to make anthrax lethal.
Cavanagh and Bobay say that knowledge of AbrB's function could make it a likely target for a drug that would knock out its function. That would prevent anthrax from "going lethal."
"We now know more about the protein that causes you to die from anthrax poisoning and a brand new way of understanding how important proteins bind to targets," Cavanagh said. "This presents a whole new paradigm for drug design in the arms race against harmful bacteria and disease."
The National Institutes of Health, the Kenan Institute for Engineering, Technology & Science and the National Institute of Environmental Health Sciences supported the study.
Provided by North Carolina State University