Vitamin B1 biosynthesis: Think Rubik's cube
(PhysOrg.com) -- A key enzyme in the biosynthesis of vitamin B1 has somehow evolved the ability to perform a complex series of some 15 to 20 steps, report two Cornell chemists.
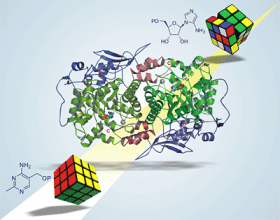
Understanding the function of the enzyme, known as HMP-P synthase, is like solving a classic Rubik's cube, said researcher Steven Ealick, because of the number of molecular rearrangements involved. But actually solving the combination will have potential implications for antibiotic development as well as for more efficient and cost effective ways of producing thiamine for food fortification, said Ealick.
Vitamin B1, which is found most widely in cereal grains, plays an essential metabolic role in all forms of life, yet its biosynthesis has not been well understood. Senior authors Ealick and Tadhg Begley, Cornell professors of chemistry and chemical biology, and first author Abhishek Chatterjee, Ph.D. '09, and colleagues report their research findings on HMP-P synthase online and in the December issue of Nature Chemical Biology (4:12).
The researchers have characterized the enzyme's structure and are poised to work out a detailed mechanism for its activity in vitamin B1, also known as thiamine, synthesis. Their structural characterization unlocks an important door toward understanding the steps needed to produce this critical molecule.
"HMP-P synthase catalyzes the most complex unresolved chemical reaction in primary metabolism," said Ealick. This reaction involves the conversion of one molecule, abbreviated AIR, into another, abbreviated HMP-P.
"If a chemist were to write a mechanism for how you get from AIR to HMP-P, it would involve 15-20 steps, and yet one enzyme -- HMP-P synthase -- catalyzes the entire reaction," Ealick said.
Defining the enzyme's structure gave Begley and Ealick the tools they need to solve the bigger puzzle: The scientists have identified how the energy needed to power such a process is produced, and more critically, they identified the enzyme's active site, where the conversion from AIR to HMP-P takes place.
Their next steps toward understanding the complex conversion include creating various mutations at this active site to stop the conversion process at different points along the pathway. From there, they hope to be able to identify intermediate molecules and experimentally work out the multistep process.
While characterizing HMP-P synthase, the researchers were surprised to learn from the enzyme's structure that it belongs to a larger superfamily of enzymes -- the radical SAM group -- that performs "heavy-duty chemistry" like the reaction that produces HMP-P.
"We didn't know this was a radical SAM enzyme until we saw that structure," said Ealick. Like other radical SAM enzymes, HMP-P synthase contains an iron-sulfur cluster that produces an unpaired electron that can initiate highly complex reactions; the problem is, this cluster is located at the opposite end of the enzyme relative to other family members, which is why a bioinformatics screen failed to identify it.
Understanding thiamine biosynthesis has several practical applications, Ealick said. Mammals do not have the ability to make thiamine and must instead ingest it, which means that pathways unique to microbial thiamine synthesis could be targets for antibiotic drug development. Additionally, instead of chemically synthesizing thiamine to fortify foods, it may eventually be possible to employ modified microorganisms as primary vitamin factories -- an advance that would greatly increase the efficiency of thiamine production while simultaneously decreasing the cost.
"Organisms are very efficient at making the molecules that they're programmed to make," said Ealick, which seems particularly true when one considers that such incredibly complex reactions can be accomplished by single enzymes.
Provided by Cornell University