Newly identified cells make fat
To understand where fat comes from, you have to start with a skinny mouse. By using such a creature, and observing the growth of fat after injections of different kinds of immature cells, scientists at the Howard Hughes Medical Institute and Rockefeller University have discovered an important fat precursor cell that may in time explain how changes in the numbers of fat cells might increase and lead to obesity. The finding, published online in this week's issue of the journal Cell, could also have implications for understanding how fat cells affect conditions such as diabetes and cardiovascular disease.
"The identification of white adipocyte progenitor cells provides a means for identifying factors that regulate the proliferation and differentiation of fat cells," says senior author Jeffrey Friedman, who is the Marilyn M. Simpson Professor at Rockefeller and a Howard Hughes Medical Institute investigator.
Obesity, a major public health problem in the United States and increasingly in much of the Western world, results, in part, from an increase in the mass and number of white fat cells. Because white fat cells are post-mitotic, meaning that they cannot divide, scientists have hypothesized that a population of fat precursor cells must exist in the fat depot in order to produce new fat cells. But identifying these fat precursor cells has been difficult.
With the assistance of researchers in Rockefeller's Flow Cytometry Resource Center, first author Matt Rodeheffer, a postdoctoral associate in Friedman's Laboratory of Molecular Genetics, used a cell sorting technique called fluorescence-activated cell sorting, or FACS, to search for cell populations that could produce fat in cell cultures and identified two such populations.
To determine if these cells could develop into fat cells in living animals, Rodeheffer injected these cell populations into the fat depots of a genetically engineered mouse, developed at NIH, called fatless, which lacks white fat and mimics a condition in humans called lipodystrophy that also results in diabetes.
Rodeheffer found that only one of the isolated cell populations, which express the CD24 cell-surface marker protein, produced fat tissue in the fatless mouse. This population normally represents only .08 percent of the non-adipocyte population in adipose tissue.
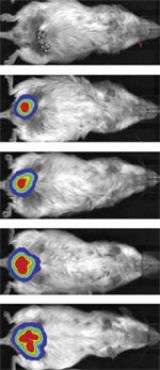
An imaging assay recently developed by co-author Kivanç Birsoy, a graduate student in Friedman's laboratory, enabled Rodeheffer to observe the CD24-expressing cells form fat in a living animal. Birsoy's technique uses another animal strain called the leptin-luciferase mouse, in which the visibly detectable marker luciferase is expressed under the control of the promoter of the gene that produces the hormone leptin. In this mouse strain the luciferase marker gene only switches on in mature fat cells, and provides a non-invasive way of watching immature fat cell precursors develop into mature fat cells in a living animal over time.
"I injected the CD24+ cells - which represent a very small population of cells in normal adipose tissue - into a site where the fat would normally develop in the fatless mouse, and I found that a normal sized fat depot forms at the site of injection," says Rodeheffer.
Rodeheffer also found that the injection of the fat-producing cells corrects the fatless mouse's diabetes, and the fat cells secrete adipocyte-specific signaling proteins called cytokines. Both of these results confirm that the cells produced in the fatless mouse are functional fat cells.
"This finding gives us a better understanding of the basic biology of adipose tissue and opens the door for us and for other researchers to be able to study these cells in living animals and determine the molecular factors that regulate formation of adipose tissue," says Rodeheffer. "We then can potentially study how the growth and differentiation of these cells are regulated in obesity and determine whether or not the molecular events that are involved in the regulation of adipose tissue are contributing factors to other pathologies, such as diabetes and cardiovascular disease, that are associated with obesity and metabolic syndrome."
Source: Rockefeller University