How the malaria parasite hijacks human red blood cells
A new study—done on a scale an order of magnitude greater than anything previously attempted in the field of malaria—has uncovered an arsenal of proteins produced by the malaria parasite that allows it to hijack and remodel human red blood cells, leaving the oxygen-carrying cells stiff and sticky. Those effects on the blood cells play a major role in the development of malaria, a disease responsible for millions of deaths every year, the researchers report in the July 11th issue of the journal Cell, a Cell Press publication.
" It's a nice piece of biology revealing how the parasite survives in and totally changes red blood cells," said Alan Cowman of The Walter and Eliza Hall Institute of Medical Research in Australia. Now that those players have been found, "there may be some way of inhibiting these processes by drugs or possibly a live vaccine."
Plasmodium falciparum causes the most severe form of malaria in humans with one to three million deaths annually, the researchers said. Once in the blood, multiplication of the parasite inside red blood cells (also known as erythrocytes) is responsible for its severity and mortality associated with the disease. After the parasite invades, the red cells undergo profound structural and morphological changes, dramatically altering their physical properties and impairing circulation. In contrast to normal red blood cells, parasitized cells are rigid and adhere to the lining of the blood vessels and other cell types.
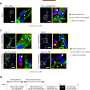
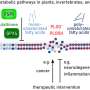
Those changes are known to be caused by proteins the parasite produces inside the cells of its host and exports across several membranes out to the red cell itself. Earlier studies showed two important ingredients: P. falciparum erythrocyte membrane protein (PfEMP1), which allows infected cells to stick to blood vessels, and knobs made up of a second protein (knob associated histidine-rich protein or KAHRP) that anchor PfEMP1 at the red cell surface.
Their screen turned up eight genes encoding proteins required for export of the PfEMP1 and assembly of knobs. Additionally, they show that multiple proteins play a role in generating increased rigidity of infected erythrocytes.
" In summary," the researchers wrote, "we have used a gene knockout approach on a scale not previously attempted in this organism to address the role of P. falciparum proteins that are exported into the parasite-infected erythrocyte. Collectively these proteins act like the secretion systems seen in bacteria in which pathogenicity arises from secreted proteins that interact with host cells by direct injection or by their presence in the extracellular milieu. …It may be valuable to adopt approaches being tested in bacteria in which these systems are the target of new therapeutic approaches aimed at minimizing pathogen virulence."
However, Cowman added, while they now know the identity of the several proteins involved, they still don't know what those proteins actually do. Those are questions Cowman's team now plans to pursue in greater detail.
Source: Cell Press