Researchers discover 'on switch' for cell death signaling mechanism
Scientists at Burnham Institute for Medical Research (Burnham) have determined the structure of the interactions between proteins that form the heart of the death inducing signaling complex (DISC), which is responsible for triggering apoptosis (programmed cell death).
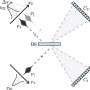
The research, performed by Stefan Riedl, Ph.D., and colleagues, published online on Dec. 31 in the journal Nature, highlights how protein-protein interactions between Fas receptor and Fas-associated death domain protein (FADD) mechanistically control DISC formation. The X-ray crystal structure of the Fas-FADD death domain complex revealed a particular arrangement of four FADD death domains bound to four Fas death domains. The structure showed that Fas undergoes a conformational change, creating an open form of the protein that acts as a site for FADD binding and also participates in the association of other Fas molecules in the clustered complex. Dr. Riedl and colleagues propose that Fas opening itself acts as a control switch for DISC formation and initiation of apoptosis.
"We found an explanation for why binding of Fas ligand is not enough to initiate DISC formation and set cell death in motion," said Dr. Riedl. "You need a special arrangement of Fas receptors to trigger opening of the Fas death domain, and only then do you get activation. Another interesting point is that this X-ray crystal structure uncovered a general mechanism for receptor signaling solely by protein clustering. Understanding the initiation of the death inducing signaling complex is of great interest because if you can activate or inhibit cell death you can have a major impact on many diseases such as cancer."
This work, by scientists of the Apoptosis & Cell Death program at the Burnham Cancer Center and their collaborators, sheds the first light on the detailed architecture of this elusive complex. Despite intense efforts by various teams, the nature of the Fas-FADD interactions and their role in DISC signaling had not been directly characterized prior to this study. The X-ray crystal structure now provides detailed information about the Fas-FADD complex at a resolution of 2.7 Angstroms. Electron microscopy studies additionally revealed that incubation of Fas death domain with full-length FADD resulted in the formation of DISC-like structures that clustered together.
Source: Burnham Institute