Scientists discover novel histone demethylase protein complex
The Stowers Institute's Workman Lab has discovered a novel histone demethylase protein complex characterized in work published today in Molecular Cell.
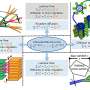
The Histone H3 protein is an important component of chromatin, the packing material wrapping up chromosomal DNA and preventing unwanted transcription of the message encoded in the DNA. Histone H3 can be altered by adding (methylating) or removing (demethylating) methyl groups from the histone protein. When genes are transcribed, parts of chromosomes are opened, making them susceptible to inappropriate use. Cells mark transcribed regions of chromosomes with a "landmark," called H3 lysine 36 methylation (H3K36), to direct appropriate use.
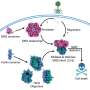
Working in fruit flies, the Workman Lab investigated how cells direct dKDM4A, a novel histone demethylase protein, to specific locations, which is important because dKDM4A is responsible for removal of landmark histone modifications during transcription elongation.
"We discovered that dKDM4A can remove specific forms of H3K36, reversing methylation and helping to regulate transcription elongation," said Chia-Hui Lin, Predoctoral Researcher and lead author on the paper. "Surprisingly, we found that dKDM4a associates with Heterochromatin Protein 1a (HP1a), a classic transcriptional silencing factor. The binding of HP1a stimulates the histone demethylation activity of dKDM4A. In fruit fly larvae without HP1a, we found a significantly increased level of H3K36."
"It is known that HP1a acts as a 'scaffold' component during transcription silencing, but recent findings of HP1a's involvement in actively transcribed regions had confounded the chromatin field," said Jerry Workman, Ph.D., Investigator and senior author on the paper. "This work suggests a possible role for HP1a in transcription activation by facilitating histone demethylation by dKDM4A to remove an important histone mark during elongation."
The discovery applies to human health especially as it relates to Huntington disease. The human enzyme that adds methylation marks on histone H3K36 interacts with the Huntington protein, which causes Huntington's disease. Additionally, the human version of dKDM4A functions as an oncogene, which has the potential to cause a normal cell to become cancerous. Overexpression of such a gene product can lead to esophageal squamous carcinoma and prostate cancer. The Workman Lab's efforts to learn more about the dynamic regulation of H3K36 methylation may lead to the discovery of potential mechanisms to cure or alleviate these diseases.
Source: Stowers Institute for Medical Research