Improved Ion Mobility Is Key to New Hydrogen Storage Compound
A materials scientist at the National Institute of Standards and Technology has deciphered the structure of a new class of materials that can store relatively large quantities of hydrogen within its crystal structure for later release. The new analysis may point to a practical hydrogen storage material for automobile fuel cells and similar applications.
The abundant element hydrogen could play a role in replacing carbon-based fuels for transportation in the future, but researchers first must develop a method to store and release large amounts of the highly flammable, odorless invisible gas economically and safely. There are materials that are known to trap relatively large quantities of hydrogen, at normal pressures, but to date they all require heating to fairly high temperatures to release the hydrogen.
Hui Wu, a research associate from the University of Maryland working in a cooperative research program at the NIST Center for Neutron Research, has been investigating a new hydrogen storage compound that mixes lithium amide with lightweight metal hydrides.
Lithium amide can hold more than 10 percent of hydrogen by weight, well above the 6 percent target set by the U.S. Department of Energy as a 2010 goal for a hydrogen storage material for transportation. The material absorbs and releases hydrogen reversibly, but both absorbing and releasing the hydrogen requires high temperatures and also produces a toxic byproduct, ammonia.
Metal hydrides also store hydrogen, though not as well, but recently it’s been shown that a combination of the two not only can store significant quantifies of hydrogen but also can release it at lower temperatures than the lithium amide alone (about 100 degrees Celsius) while generating much less ammonia.
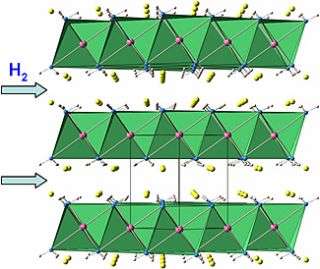
To understand how the compound achieves this, Wu used neutron analysis to work out the atomic structure of the material, which she found consists of layers of calcium between which lithium ions travel rapidly. The easy travel allows the material to transfer the hydrogen at lower temperatures. Also the hydrogen ions in the amide and hydride mixture combine easily and release hydrogen at lower temperature without creating much ammonia.
“I found that the mobility of small ions in the mixed amide-hydride system greatly improves hydrogen storage properties,” Wu explains. “This finding helps us understand how hydrogen travels in and out of these systems and that may lead to a rational development of better materials for hydrogen storage.”
Citation: H. Wu. Structure of ternary imide Li2Ca(NH)2 and hydrogen storage mechanisms in amide-hydride system. Journal of the American Chemical Society ASAP Article, Web release date: April 30, 2008
Source: NIST