Size matters – to the immune system, at least
A theory about the way in which the immune system identifies and responds to invasion has been confirmed by Oxford researchers in a paper published in Nature today. The paper shows that a highly sensitive and specific immune response hinges on something as straightforward as large and small molecules jostling into size order.
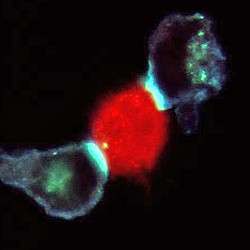
Picture: an infected cell is examined by two T-cells simultaneously. The bright areas of colour at the cell interfaces are areas where the T-cell receptors are gathering
T-cells are a crucial part of our immune system: they patrol the body surveying cells and triggering the immune system to attack those that are infected. The surface of every cell, including T-cells and infected cells, is covered in a variety of molecules. When a T-cell comes into contact with an infected cell, a receptor molecule on the T-cell’s surface will bind with a telltale molecule on the surface of the infected cell which flags up the infection (called a peptide MHC). This binding triggers the immune response.
Researchers have long wondered how exactly this triggering works. T-cells are kept in check by a constant battle at their surface between the small molecules that trigger the immune response and large, long molecules such as CD45s which prevent the immune response from being switched on. The spoilsport CD45 plays a crucial role: the immune system can attack as well as protect the body, and allergies and serious autoimmune illnesses are the results of the immune system being activated inappropriately. How, though, do T-cells overcome the inhibitory effect of CD45s when they do need to launch an attack on invaders?
The answer is now shown to be the kinetic segregation model, conceived at Oxford 10 years ago by Professors Anton van der Merwe and Simon Davies, and shown to be correct in today’s Nature paper, on which Professor van der Merwe is an author along with three other members of Oxford’s Sir William Dunn School of Pathology and a scientist at Imperial College London. The model proposes that, because both T-cell receptors and the telltale peptide MHCs are relatively small molecules, they can only come into contact and bind when the bulkier CD45s are out of the way – in which case the immune response can be activated without impediment.
Molecules move around freely on the cell surface, and this constant reconfiguration means it is likely that groups of smaller molecules will spontaneously come together at some point. When these ‘clearings’ of shorter molecules occur on two adjacent cells, a ‘close contact zone’ between the two cells is formed, where the small molecules on each cell can come into direct contact with each other, rather than being held ‘at arm’s length’ by the presence of the larger molecules. The close proximity allows a T-cell receptor molecule to bind with any peptide MHC (that ‘flag’ for infection) that it finds. The bulky inhibitory molecules cannot enter the zone – which becomes fixed once the immune response begins – and so can no longer halt the immune response.
The researchers tested this theory by using long versions of the peptide MHCs, genetically engineered by Dr Keith Gould of Imperial College London and further modified by first author on the paper Dr Kaushik Choudhuri from Oxford. They found that these long peptide MHCs still bound to the T-cell receptors, but, as predicted, the immune response was not triggered.
David Wiseman from Oxford’s Sir William Dunn School of Pathology, one of the authors of the paper, said: ‘By looking at the cells with an electron microscope we could actually see that the space between the cells was wider when we used the longer peptide MHCs. In that situation, the CD45s wanting to switch the immune response off were no longer being segregated out.’
To check that the results were genuinely because of the size difference rather than any structural differences, the team did control experiments on completely different molecules with similar dimensions, proving that it was size that was important, not structure. The mechanism may well prove to apply not just to the immune system but to any system where cells meet and interact, which would encompass the nervous system and other crucial processes in the body.
The work was funded primarily by the Medical Research Council, while Dr Choudhuri was supported by the Wellcome Trust.
Mr Wiseman said: ‘What we have shown is a whole new way that cells can control themselves. These are the earliest events in T-cell triggering, the key activation step in immune surveillance, and all it comes down to is whopping great molecules being shut out of close contacts zones, simply by virtue of their whopping greatness.’
Source: University of Oxford