New study furthers development of drugs targeting potassium-ion channels
In the field of drug development, frequent targets are cell-membrane proteins, such as ion channels, tiny pores that allow various substances to enter and exit the cell. Ion channels, which govern the passage of sodium, potassium, and calcium ions, play a key role in many physiological functions, such as the nerve impulse (and hence the entire nervous system), muscle contraction, and insulin secretion.
Despite the fundamental importance of ion channels to larger-scale activities in the body, scientists do not have a full understanding of how these channels work. To develop new drugs – say, for treating nervous system or cardiovascular disorders – these knowledge gaps must be filled.
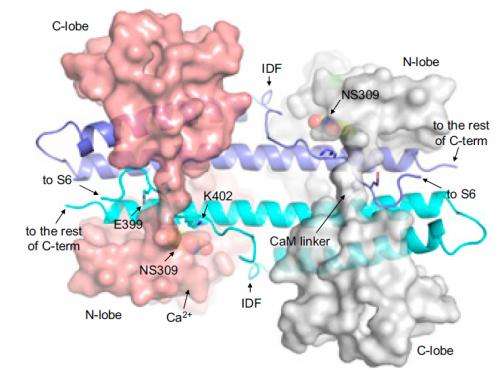
A research group from Thomas Jefferson University in Philadelphia has recently taken a step in this direction by investigating a variety of potassium-ion (K+) channel that is activated by calcium ions (Ca2+). They describe a particular channel fragment – a target for pharmaceuticals – that is "intrinsically disordered" (ID), meaning it takes on many shapes, or "conformations." This has made it difficult to study with standard methods like x-ray crystallography, a synchrotron x-ray technique used to gather structural information from a protein crystal. But the researchers show that the ID fragment stabilizes when in complex with a potential drug known as NS309, a calcium-ion-sensing protein called calmodulin, and the channel's calmodulin binding domain.
Like most potassium-ion channels, the Ca2+-activated K+ channel is a tetramer (a large four-part molecule), with each part consisting of six transmembrane domains that change conformation to allow the channel to open. It is classified as a "small-to-intermediate conductance" K+ channel (abbreviated as SK or IK channels) because of the modest rate of ions passing through it. When calcium ions bind to the calmodulin (short for calcium modulated protein and abbreviated CaM) at the site of the channel's calmodulin binding domain (CaMBD), it causes a cascade of events and conformational changes that open the channel. But more specific details of this process have been lacking.
"Given the roles of SK/IK channels in physiological and pathophysiological conditions, a tremendous amount of effort has been devoted to developing small molecules, including NS309, that target them," said Ji-fang Zhang, a scientist in the Jefferson Medical Center's Department of Molecular Physiology & Biophysics and an author on the paper. "However, it has not been known how these compounds achieve their effects in potentiating SK/IK channels."
Zhang and his colleagues worked at several facilities, including the National Synchrotron Light Source (beamlines X29A and X6A) at Brookhaven National Laboratory and at the Advanced Light Source at Lawrence Berkeley National Laboratory. They first studied a crystal of CaM and the CaMBD in complex and noticed that a protein fragment was missing from the structure. That it was missing from the x-ray diffraction data suggested that it was intrinsically disordered. Yet when the group studied a crystal that also contained NS309, the fragment became visible and took on a well-defined shape.
In this distinctive conformation, the group discovered, the ID fragment led to an increased probability that the channel will open given a certain concentration of calcium ions, which the group confirmed via several experiments. In this respect, the ID plays a unique role in coupling Ca2+ sensing by CaM and in the mechanical opening of SK channels.
"Our results provide direct evidence that NS309 can stabilize the conformation of the CaM-SK complex, which favors channel opening," said Zhang.
More information: "Unstructured to structured transition of an intrinsically disordered protein peptide in coupling Ca2+-sensing and SK channel activation," Miao Zhang, John M. Pascal, and Ji-Fang Zhang. PNAS. DOI: 10.1073/pnas.1220253110
Journal information: Proceedings of the National Academy of Sciences
Provided by Brookhaven National Laboratory
















