Study reveals little-known cell networks vital to circadian rhythm
Circadian rhythm is the basic 24-hour cycle that involves various behaviors, including sleeping and eating, in all living organisms. In mammals, the circadian clock is organized hierarchically in a series of multiple oscillators. At the top of this hierarchy, the suprachiasmatic nucleus (SCN), a region of the brain that is the body"s main rhythmic regulator, integrates light information from the eyes and coordinates peripheral oscillators throughout the body.
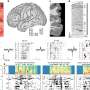
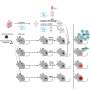
By examining effects of genetic mutations at the level of single cells and tissues, the study showed that intercellular mechanisms are in fact essential to the operation of cellular circadian clocks.
"Our study reveals some previously overlooked mechanisms for sustaining cellular circadian rhythm," said Steve A. Kay, whose laboratory spearheaded the research. "Essentially, when cells communicate en masse through these highly networked electrical or neurochemical interactions, the system responds far more effectively."
The SCN intercellular network, Kay said, is necessary not only to stabilize oscillators in the peripheral tissues but also to provide a robust response to various genetic mutations. In fact, the network interactions unique to the SCN can compensate for some genetic defects in the Period (Per) and Cryptochrome (Cry) genes-the clock genes-to preserve circadian rhythm.
In fact, the circadian defects observed in mutant oscillators were clearly more extreme when measured at the tissue and cell levels than demonstrated by behavioral observations.
"Because single cells are ordinarily capable of functioning as autonomous oscillators," Kay noted, "our previous understanding of clock mechanisms has rested precariously on the idea that if we studied behavior, we could assume that same thing was happening at the single cell level. Our study shows that's not the case."
The lack of networked interactions in peripheral tissues may actually be an adaptive feature in most circumstances. SCN cells in vivo must synchronize not only to light-dark cycles but also to one another to coordinate circadian behavior. Lack of coupling may allow peripheral oscillators to anticipate and respond rapidly not only to the synchronizing cues emanating from the SCN but also to physiological signals related to feeding and behavior.
"Future studies should focus on addressing the system impact of these cellular networks," Kay said. "Our results validate clock model predictions previously overlooked or sometimes regarded as model flaws. Newer models are needed to accommodate the novel cell-autonomous phenotypes we uncovered."
Source: Scripps Research Institute