Natural gut hormone offers hope for new obesity drug
A hormone found naturally in the gut is the basis of a new drug to tackle obesity, one of three inaugural awards under the Wellcome Trust's Seeding Drug Discovery initiative. The drug is being developed by one of the world's leading obesity experts, Professor Steve Bloom at Imperial College London's Hammersmith Hospital campus.
"Over 30,000 deaths a year are caused by obesity in England alone, so there is a clear need to develop a treatment to tackle this problem," says Dr Ted Bianco, Director of Technology Transfer at the Wellcome Trust. "Yet this need for effective anti-obesity therapies is currently unmet. We believe that Professor Bloom's research holds great promise and, with our support, can be translated into tangible benefits to health."
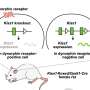
Recent research by Professor Bloom and his team identified the role played by gut hormones in appetite control. These hormones are released when a person eats, acting as neurotransmitters to indicate to the brain to stop eating. In particular, the researchers are interested in pancreatic polypeptide (PP), which they believe may provide a solution to appetite suppression and is the most likely candidate for translating into a treatment.
"Developing a treatment based on natural appetite suppression, mimicking our body's response to being full, has the potential to be safe and effective," says Professor Bloom. "We believe that pancreatic polypeptide may be the answer."
Professor Bloom points to research showing that people with benign PP-secreting tumours have elevated levels of the hormone and yet appear to show no adverse side-effects.
"These people may have had high levels of PP for ten or fifteen years without showing side effects," he explains. "In that sense, they have provided us with a natural experiment that suggests that excess levels of PP over a long period are safe. It does not appear to raise blood pressure or heart rate, or any other obvious side effects."
With funding from the Seeding Drug Discovery initiative, Professor Bloom and colleague Dr Caroline Small hope to develop a synthetic form of PP which can be administered to patients.
"The trouble with PP is that it would need to be injected daily and cannot be taken as a pill," says Dr Small. "Naturally, this is not very convenient, so we need to develop an injectable form that is longer lasting and can be administered on a weekly basis to make it more practical."
If successful, the proposed research may lead to a treatment within five to eight years.
"It is likely that if we are successful, the treatment may be fast tracked to meet the urgent demand to tackle the obesity crisis," she explains. "There is currently a lack of effective treatments and our proposed drug is based on a natural way of controlling the body's appetite, which makes it more attractive."
Source: Wellcome Trust