Protein's effects essential for kidney-to-bladder urine transfer
Tests of a protein's role in the immune system have revealed a surprising connection to a kidney problem that occurs in approximately one percent of all live births.
"This condition, which is known as functional obstruction, impairs the ureter's ability to pump urine from the kidney to the bladder," says senior author Wojciech Swat, Ph.D., assistant professor of pathology and immunology. "If untreated, this leaves urine stuck in the kidney, which balloons and becomes at risk of failure."
Swat and colleagues found a similar condition in mice after the gene for a protein known as discs-large homolog 1 (DLGH1) was disabled.
When properly diagnosed, functional obstruction is normally treatable with surgery. But the study also produced tentative evidence that DLGH1 dysfunction may affect long-term risk of kidney failure even after successful treatment.
"We noted about one-third fewer nephrons in mice where this gene had been completely disabled," says co-senior author Jeffrey H. Miner, Ph.D., associate professor of medicine and of cell biology and physiology. "Nephrons are the filtering units of the kidney, and having too few of them can predispose to kidney failure later in life."
The findings, published in the online edition of Proceedings of the National Academy of Sciences, are the first to identify a factor that guides the orientation of smooth muscle cells, which surround the ureter and provide the pumping action that brings urine from the kidney to the bladder. Smooth muscles also play important roles in swallowing, digestion, reproduction, respiration, vision and other biological processes.
Swat and postdoctoral fellow Bénédicte Sammut, Ph.D., originally generated mice lacking DLGH1 to study the protein's role in a structure known as the immune synapse. As conceived by Swat's colleague Andrey Shaw, M.D., the Emil R. Unanue Professor of Immunobiology, the immune synapse is a specialized communication structure created by two immune cells. According to the theory, this communication activates immune cells to fight invaders. Swat had hypothesized that DLGH1 might help move structures to one side of an immune cell for creation of the synapse.
As expected, loss of DLGH1 proved fatal for mice during or shortly after birth. While examining the mice, Swat and co-author Thaddeus Stappenbeck, M.D., Ph.D., assistant professor of pathology and immunology and of molecular biology and pharmacology, noticed that many had swollen kidneys.
"We didn't think the kidney problems were what was killing them, but we were told to go to Jeff Miner for expert advice on what was happening," Swat says. "That's the beauty of Washington University--that it's so easy to collaborate across different research specialties."
With help from other colleagues, Swat, Miner, and graduate student Zhen Mahoney were able to arrange for video microscopy of mouse ureters in action. These revealed that peristalsis, the normal pulse-like contraction that squeezes the ureter at one point while opening it up at the next point in the tube, had been disabled.
"We saw that peristalsis had been replaced by a vertical, non-squeezing motion that went down the ureter but propelled very little urine through it," Swat says.
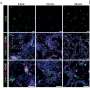
A closer look at the musculature surrounding the ureter revealed why. Normally the ureter is surrounded by two layers of smooth muscle: an outer, longitudinal sheath where the muscles cells are oriented along the path of the ureter; and an inner, circular layer where the cells form rings around the tube of the ureter.
"In mutant mice, the longitudinal muscle is fine, but the circular muscle is either missing or has become longitudinal," Miner says.
Swat and Miner are planning to collaborate with geneticists to see if functional obstruction in humans can be linked to alterations in DLGH1, a possibility Swat calls "extremely likely."
"Very little is known about the development of smooth muscle, which is important to so many areas of biology," Swat says. "So not only are the clinical implications important, we're also breaking new ground on some interesting basic research issues."
Follow-up studies will determine the origin of the DLGH1 that helps orient cells in the circular layer. Those cells could be making the DLGH1 themselves, or they may be responding to cues from other cells that make the protein.
"We have to answer that question before we can get serious about figuring out how this process works at a molecular level," Swat says.
Source: Washington University School of Medicine