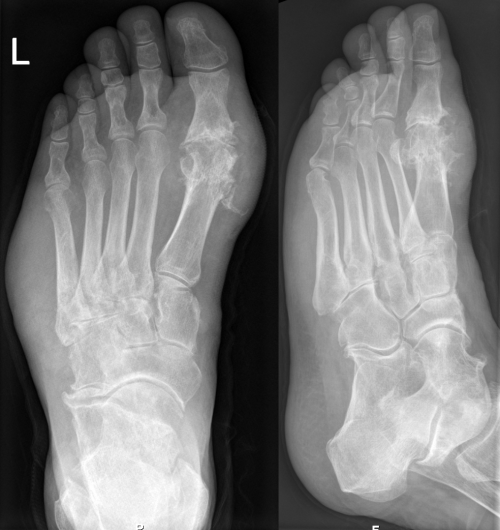
Gout in X-ray of left foot. Credit: Hellerhoff/Wikipedia.
(Medical Xpress)—Apes, including humans, lack an enzyme called uricase that breaks down uric acid. Because we lack uricase, we are predisposed to developing gout. After reconstructing ancient versions of the enzyme found in other mammals, Eric Gaucher of the Georgia Institute of Technology and his colleagues have concluded that apes lost the ability to produce uricase so they could better convert fructose, fruit sugar, to fat for survival when fruit was scarce. The research appears in the Proceedings of the National Academy of Sciences.
Gout is a painful condition caused by uric acid crystals building up in the joints. It occurs when there is so much uric acid in the blood the kidneys can't process it all. Most mammals have an enzyme called uricase that breaks down uric acid into substances that are easier to excrete. In apes, however, a mutation makes the gene that produces uricase ineffective. Consequently, apes have three to ten times more uric acid in their blood than other mammals.
Gaucher and his team wanted to understand how the ability to produce uricase changed over the course of mammalian evolution. They reconstructed ancient versions of the enzyme, starting with that produced by the last common ancestor of mammals about 90 million years ago. The researchers found that over time, mutations in the gene that codes for uricase made the enzyme progressively less effective. Finally, about 17 million years ago, apes developed a mutation that causes us not to produce any uricase at all.
The team noticed that the greatest reduction in uricase's effectiveness took place when the Earth was cooling. From this, they hypothesized a connection between the decreasing ability to metabolize uric acid and an increasing scarcity of fruit. Uric acid is a byproduct of the breakdown of fructose and stimulates us to create fat stores. As the climate cooled and fruit became harder to find in winter, high levels of uric acid would have made it easier to store fat and therefore made survival more likely. To test their hypothesis, the researchers added ancient versions of uricase to human cells and then examined how these cells responded to fructose. They discovered that the cells produced less fat.
Gaucher's team think their discovery could provide support for the "thrifty gene" hypothesis, which says that the propensity for modern-day humans to become obese comes from our ancestors' need to store fat efficiently to prepare for times of scarcity. Previous research shows an association between high levels of uric acid and metabolic syndrome, a condition associated with obesity. The team believes reengineering ancient forms of uricase for use in humans would have therapeutic benefits.
More information: "Evolutionary history and metabolic insights of ancient mammalian uricases," by James T. Kratzer et al. PNAS, www.pnas.org/cgi/doi/10.1073/pnas.1320393111
Journal information: Proceedings of the National Academy of Sciences
© 2014 Medical Xpress