FDA has safety concerns on Merck insomnia drug
Federal health regulators say an experimental insomnia drug from Merck can help patients fall asleep, but it also carries worrisome side effects, including daytime drowsiness and suicidal thinking.
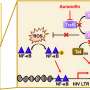
The Food and Drug Administration released its review of the company's sleep aid, suvorexant, ahead of a public meeting on Wednesday. The pill works by temporarily blocking chemical messengers that keep people awake.
The FDA said company trials show suvorexant was better than placebo at helping people fall asleep and stay asleep. Regulators said Monday the drug's effectiveness was consistent across several doses tested by Merck & Co.
But patients taking the highest dose of the drug showed an eight-fold increase in daytime drowsiness, which could interfere with driving the next morning. Suvorexant was also associated with increased risk of suicidal thinking.
© 2013 The Associated Press. All rights reserved.