Team finds promising new drug target for Alzheimer's disease
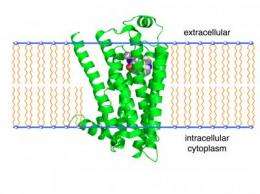
Researchers at the University of Illinois have identified a potential drug target for the treatment of Alzheimer's disease: a receptor that is embedded in the membrane of neurons and other cells.
A protein fragment associated with Alzheimer's disease activates this receptor, sparking increased activity in the affected neurons, eventually leading to cell death, the researchers report. The new findings appear in the FASEB Journal.
Scientists have known for decades that a protein fragment, called amyloid-beta (AM-uh-loyd BAIT-uh), is a key to the riddle of Alzheimer's disease. Alois Alzheimer himself first found aggregates of this "peculiar substance" in the brain of a dementia patient after her death. These bundles of protein, or plaques, are composed almost entirely of amyloid-beta, and still are used to diagnose Alzheimer's disease after death.
Animals with amyloid plaques in the brain experience a decline in brain function that mirrors that of Alzheimer's disease. A recent study found that neurons closest to these plaques tend to be hyperexcitable relative to normal, while activity in the surrounding neurons is depressed, indicating an imbalance in brain activity associated with these plaques.
Other studies have found that clumps of only two, or a few, amyloid-beta fragments somehow stimulate a receptor, called the AMPA receptor. When amyloid-beta binds to a neuron, the AMPA receptor opens a channel that lets calcium or sodium ions into the cell.
Normally the AMPA receptor opens this channel only when it binds to glutamate, a potent neurotransmitter that is important to normal brain function as well as memory and learning. In either case, the quick influx of ions causes a nerve impulse.
To date, scientists have not been able to identify a mechanism by which amyloid-beta causes the AMPA receptor channel to open, however.
"If a mouse is exposed to amyloid-beta in the brain, it impairs neuron function, causing memory deficits and behavioral deficits," said Kevin Xiang, a professor of molecular and integrative physiology at Illinois who led the new study with professor Charles Cox and postdoctoral fellows Dayong Wang and Govindaiah in the same department. "The question is how this peptide causes all these detrimental cellular effects."
For the new study, the researchers focused on the beta-2 adrenergic receptor, a protein that - like the AMPA receptor - resides in the cell membrane. Neurotransmitters and hormones normally activate the beta-2 adrenergic receptor, but amyloid-beta also induces a cascade of events in the neuron by activating the beta-2 adrenergic receptor, the researchers found. One of the downstream effects of this interaction is activation of the AMPA receptor ion channels. (In mice lacking the beta-2 adrenergic receptor, amyloid-beta had no discernible effect on AMPA receptors, they found.)
"We showed that we needed the presence of beta-2 adrenergic receptors to get the increase in the AMPA-mediated response," Cox said.
Further experiments showed that amyloid-beta does bind to the beta-2 adrenergic receptor.
Previous studies had found that blocking the AMPA receptor could alleviate the misfiring caused by amyloid plaques in the brain. But the AMPA receptor, which responds to glutamate, is important to learning and memory, so blocking it could also do harm, the researchers said.
"Glutamate is such a ubiquitous neurotransmitter throughout the brain, you can't simply go in and block its actions because if you do, you can just start rounding up the side effects," Cox said.
"Once you block the AMPA receptor you're basically dampening widespread neuronal excitability throughout the whole brain," Cox said. "Now we have something a bit more specific to latch onto: the beta-2 adrenergic receptor."
This receptor offers an attractive alternative target because, the researchers found, amyloid-beta binds to a different part of the receptor than that normally engaged by neurotransmitters and hormones. This means it may be possible to stop amyloid-beta from binding to it without hindering the other functions of the beta-2 adrenergic receptor.
Previous studies have shown that Alzheimer's patients who also take beta-blockers tend to see a slower decline in their mental function. These drugs are meant to treat hypertension and other conditions by targeting beta-adrenergic receptors, including beta-2. This finding provides further support to the idea that the beta-2 adrenergic receptor is a key to the ill effects of Alzheimer's disease.
Xiang and Cox stress that the beta-2 adrenergic receptor is almost certainly not the only important player in the damage that occurs in an Alzheimer's-afflicted brain. But they see it as a promising new potential target for future drug research.
More information: The paper, "Binding of amyloid peptide to adrenergic receptor induces PKA-dependent AMPA receptor hyperactivity," is available at: www.fasebj.org/cgi/rapidpdf/fj.10-156661v1.pdf














