FDA reviews update to Pfizer vaccine for kids
(AP) -- Federal health officials on Monday questioned whether to approve an updated version of Pfizer's best-selling anti-infection vaccine for children, despite company studies that failed to meet certain goals.
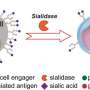
The Prevnar 13 vaccine reduces risk of infection by 13 varieties of pneumococcal disease, which causes ear infections, meningitis and pneumonia. The new version of the vaccine protects against six more varieties of the disease.
But Food and Drug Administration reviewers said that company studies failed to meet the preselected goals for three types of the disease, according to documents posted online.
The missed goals were statistical measures for vaccine response selected by the FDA. However, since no other vaccine fights the same strains of the disease as Prevnar, the FDA said the significance of the missed goals "may not be clear."
Dr. Emilio Emini, Pfizer's chief of vaccine research, said the missed goals were due to statistical groupings of patients in the study, not a flaw with the vaccine itself.
Pfizer's briefing documents note that the World Health Organization's vaccine guidelines say meeting the study goals in question is "desirable, but not an absolute requirement."
The FDA will ask a panel of outside vaccine experts to weigh in on the results at a meeting Wednesday. The agency is expected to make its decision on the vaccine by Dec. 30. The FDA is not required to follow the group's advice, though it usually does.
Pfizer acquired Prevnar from Wyeth, as part of a $68 billion buyout that closed last month. Prevnar's $2.7 billion in sales last year trailed only those of the antidepressant Effexor for Wyeth.
Wyeth applied for approval of Prevnar 13 in March and was granted a priority review from the FDA, which indicates the agency sees the product as a public health priority.
Pfizer has said the new vaccine will save lives in the U.S. and in developing countries.
"The public health imperative for this vaccine is clearly there," Emini said. "Pneumococcal disease is the number one cause of vaccine-preventable death in world."
New York-based Pfizer is seeking FDA approval to market the vaccine for infants and young children and has tested it in more than 7,000 youngsters in 13 major studies.
Prevnar has been on the market for more than nine years and is on sale in 95 countries. More than 235 million doses have been distributed, according to the company.
The vaccine requires a series of four injections, generally given at 2, 4 and 6 months old and then between 12 and 15 months old.
Prevnar 13 also recently received a positive review from a committee of European regulators, setting the stage for approval across the European Union.
©2009 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.