TB vaccine enters new clinical trials
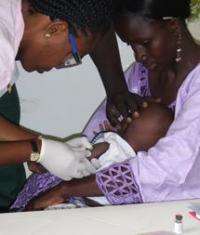
(PhysOrg.com) -- The world’s leading candidate for a tuberculosis vaccine, developed at the University of Oxford, is to enter Phase IIb proof-of-concept clinical trials, making it the first TB candidate vaccine for more than 80 years to get to this advanced stage of clinical trials in infants.
More than two billion people are infected with tuberculosis (TB) - approximately one out of every three people on the planet - and 1.8 million die annually from the disease.
Oxford researchers have developed a promising new vaccine against TB. The Oxford-Emergent Tuberculosis Consortium Ltd, a joint venture between the University of Oxford and Emergent BioSolutions Inc, is taking the vaccine forward.
The announcement of the next phase of trials was made today by the Aeras Global TB Vaccine Foundation, the Oxford-Emergent Tuberculosis Consortium Ltd, Isis Innovation, the Wellcome Trust and the University of Cape Town (UCT). The study will be conducted in South Africa, around 100 km from Cape Town, by the South African Tuberculosis Vaccine Initiative (SATVI) of UCT.
A new vaccine is urgently needed, as BCG is currently the only available vaccine against TB, and provides only variable protection against pulmonary tuberculosis, which accounts for most of the worldwide disease burden.
The trial will enrol 2,784 children less than one year of age, all of whom have received BCG at birth. It is expected that the trial will generate important safety, immunogenicity and preliminary efficacy data about the vaccine candidate.
The Aeras Global TB Vaccine Foundation is working with the Consortium to develop the vaccine, called MVA85A/AERAS-485, with additional funding from the Wellcome Trust. The vaccine candidate was originally developed at the University of Oxford by Dr Helen McShane, a Wellcome Trust Senior Clinical Research Fellow, working with Dr Sarah Gilbert, a Reader in Vaccinology, and Professor Adrian Hill, a Wellcome Trust Principal Research Fellow. It was licensed by Isis Innovation, the University’s technology transfer company to the Oxford-Emergent Tuberculosis Consortium in July 2008. The vaccine has been awarded orphan drug status by the European Medicines Agency (EMEA) and is the most clinically advanced of a new generation of tuberculosis vaccine candidates.
‘We believe this is the most exciting advance in the field of TB vaccines for over 80 years,’ said Dr Helen McShane of the Jenner Institute, University of Oxford, ‘and it is a testament to the commitment shown by the partners and funders involved. We have shown that this vaccine is safe and stimulates strong immune responses. This trial will hopefully show that the vaccine can protect people from getting TB and enable the global community to begin to control this devastating disease.’
'The search for a new TB vaccine is a complex and challenging process requiring a broad commitment and we are pleased to be collaborating with so many dedicated and talented researchers on this important effort,’ said Jerald C Sadoff, MD, President & CEO of the Aeras Global TB Vaccine Foundation. ‘There is still a long road ahead, but this marks an important milestone toward the goal of a more effective TB vaccine.’
The Aeras Global TB Vaccine Foundation is a non-profit organization working as a Product Development Partnership to develop new tuberculosis vaccines and ensure that they are distributed to all who need them around the world. Aeras collaborates with academia, industry, foundations and governments to develop new TB vaccine candidates and delivery systems, manufacture vaccines at low cost and establish intellectual property rights to assure their future availability and affordability. Aeras is funded by the Bill & Melinda Gates Foundation, the Netherlands Ministry of Foreign Affairs, the Danish International Development Agency, the Research Council of Norway and the U.S. Centers for Disease Control and Prevention.
The Oxford-Emergent Tuberculosis Consortium Ltd is a joint venture between the University of Oxford and Emergent Product Development UK Ltd. The Consortium was formed with the aim of developing the MVA85A TB vaccine to meet both developed and developing country health needs.
Provided by Oxford University (news : web)
















