Combining targeted therapy drugs may treat previously resistant tumors
A team of cancer researchers from several Boston academic medical centers has discovered a potential treatment for a group of tumors that have resisted previous targeted therapy approaches.
In their Nature Medicine report, which is receiving early online release, investigators from Dana-Farber Cancer Institute (DFCI), Massachusetts General Hospital (MGH) Cancer Center, and Beth Israel Deaconess Medical Center (BIDMC) Cancer Center report that combining two different kinase inhibitors – drugs that interfere with specific cell-growth pathways – led to significant tumor shrinkage in mice with lung cancer driven by mutations in the K-Ras gene. In addition to their association with nearly 30 percent of cases of non-small-cell lung cancer – the leading cause of cancer deaths in the U.S. – K-Ras mutations are involved in many cases of colon cancer and most pancreatic cancers, which are extremely resistant to treatment.
"Finding a way to effectively treat K-Ras-mutated cancers would be a huge advance in solid tumor oncology, since these mutations are common in several incurable cancers," says Jeffrey Engelman, MD, PhD, of the MGH Cancer Center, one of the report's co-lead authors. "Cancers with K-Ras mutations have been resistant to all targeted therapies to date, and it is exciting to learn that a combination of PI3K and MEK inhibitors, two families of drugs currently in clinical development, may be highly effective in these cancers."
The current study began with a focus on the PI3K signaling pathway, which is key to cell survival and known to control cellular motility and adhesion. PI3K mutations have caused tumor development in laboratory studies, but their role had not yet been studied in an animal model. The research team developed a transgenic mouse in which administration of the drug doxycycline induces the expression of cancer-associated PI3K mutations, leading to development of lung tumors.
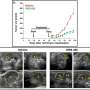
Treatment of those animals with an investigational PI3K inhibitor did lead to rapid tumor regression. Since previous studies suggested that PI3K inhibition might also block K-Ras-induced tumor development, the investigators also tested the PI3K inhibitor in mice with K-Ras-stimulated tumors. That treatment was ineffective, but since K-Ras also activates the MEK/ERK signalling pathway, the researchers treated the animals with an investigational MEK inhibitor and with a combination of both drugs. Treatment with the MEK inhibitor alone caused only a modest reduction in tumor size, but combined treatment with both agents caused the K-Ras-stimulated lung tumors to virtually disappear.
"For several years we have known that K-Ras activates two major pathways – the PI3K pathway and the MEK/MAPK pathway – and that these pathways have many redundant functions in tumor growth and survival," says Lewis Cantley, PhD, of the BIDMC Cancer Center, one of the study's co-corresponding authors. "Inhibitors of both of these pathways are now in clinical trials, and in this paper we show that, while either agent alone has a minor effect on K-Ras-driven tumors in mice, combining inhibitors of both pathways eradicates these tumors with minimal toxicity."
Kwok-Kin Wong, MD, PhD, of DFCI, also a co-corresponding author, adds, "The results of our study are truly remarkable and provide a strong and compelling scientific rationale to test this combination therapy in human phase 1 and 2 trials. This work would not have been possible without the highly productive collaboration between our laboratories at Mass. General, Beth Israel-Deaconess and Dana-Farber." Wong is an assistant professor of Medicine at Harvard Medical School, where Engelman is also an assistant professor in Medicine, and Cantley is the Castle Professor of Medicine.
The researchers are hoping to advance towards clinical trials by testing combination therapy against other models of K-Ras-mutated cancer, including those that involve additional mutations in other tumor-associated genes, and to investigate whether K-Ras-associated tumors will become resistant to combination therapy, a problem that has plagued other targeted cancer therapies.
Source: Massachusetts General Hospital