New approach to gene therapy may shrink brain tumors, prevent their spread
Massachusetts General Hospital (MGH) researchers are investigating a new approach to gene therapy for brain tumors – delivering a cancer-fighting gene to normal brain tissue around the tumor to keep it from spreading. An animal study published in the journal Molecular Therapy, the first to test the feasibility of such an approach, found that inducing mouse brain cells to secrete human interferon-beta suppressed and eliminated growth of human glioblastoma cells implanted nearby.
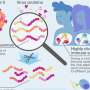
"We had hypothesized that genetically engineering normal tissue surrounding a tumor could create a zone of resistance – a microenvironment that prevents the growth or spread of the tumor," says Miguel Sena-Esteves, PhD, of the MGH Neuroscience Center, the study's senior author. "This proof of principle study shows that this could be a highly effective approach, although there are many additional questions that need to be investigated."
Glioblastoma is the most common and deadly form of brain tumor. Human clinical trials of other gene therapies have not significantly reduced tumor progression. One problem has been that patients' immune systems target the viral vectors used to deliver cancer-eliminating genes. Another issue has been inefficient gene delivery, due in part to the inherent cellular diversity found within an individual patient's tumor as well as among tumors from different patients. In addition, if tumor cells are successfully induced to express an anticancer protein, production of that protein will drop as the tumor dies, allowing any cells that did not receive the gene to resume growing. In the current study the MGH team examined whether expression of a therapeutic gene in normal brain cells could form a stable and effective anti-tumor reservoir.
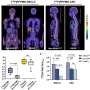
The researchers first pretreated immune-deficient mice by delivering a gene for human interferon-beta – a protein being tested against several types of cancer – into the animals' brains using adeno-associated virus vectors known to effectively deliver genes to neurons in the brain without the immune reaction produced by other vectors. Two weeks later, human glioblastoma cells were injected into the same or adjacent areas of the animal's brains. After only four days, mice expressing interferon-beta had significantly smaller tumors than did a control group pretreated with gene-free vector. Two weeks after the glioblastoma cells were introduced, the tumors had completely disappeared from the brains of the gene-therapy-treated mice.
Several additional experiments verified that the anti-tumor effect was produced by expression of interferon-beta in normal tissue. The same tumor growth suppression was seen when the genes were delivered to one side of the brain and tumor cells were injected into the other. Using a specialized vector that allows genes to be expressed only in neuronal cells and not the glial cells from which glioblastomas originate also produced similar results. While other gene therapy studies that have induced tumor regression in mouse models required several vector injections, these experiments were able to suppress growth and eliminate the implanted tumor with a single injection of the interferon-beta-encoding vector, underscoring the approach's effectiveness.
"These results are particularly important as we build on our understanding of the microenvironments that allow tumors to grow and spread," explains Sena-Esteves, an assistant professor of Neurology at Harvard Medical School. "The therapeutic principle of genetically engineering normal brain tissue could be used to manipulate proteins required for that microenvironment, preventing tumors from migrating within the patients brain and escaping other therapies." The same zone-of-resistance approach could also be applied to the treatment of other solid tumors, he notes.
Since interferon-beta treatment is known to have side effects, it will be important to identify any toxicity caused by long term secretion of the protein in the brain and develop preventive strategies, such as turning off the introduced genes. Next the MGH team is planning to test this strategy on glioblastomas that occur naturally in dogs, which could not only generate additional data supporting human trials but also develop veterinary treatments for canine patients.
Source: Massachusetts General Hospital