Heart defibrillator company signs decree
The U.S. Food and Drug Administration said Medtronic Inc. has agreed to comply with FDA rules in manufacturing its external heart defibrillators.
The FDA said Medtronic, its Physio-Control Inc. subsidiary and two high-ranking executives signed a consent decree calling for a permanent injunction involving the company's automatic external defibrillators manufactured by Physio-Control.
"The consent decree prohibits the manufacture, distribution and export of specified AEDs at or from Physio-Control's facility in Redmond, Wash., until the devices and facilities have been shown to be in compliance with the Food and Drug Administration's current 'Good Manufacturing Practice' requirements, as set forth in the … regulation for devices," the FDA said in a statement.
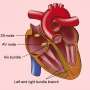
AEDs are portable devices used to restore normal heart rhythm to people who suffer heart attacks that produce ventricular fibrillation. The AEDs deliver an electric shock that stuns the heart for a moment, giving it the chance to resume beating effectively.
FDA inspections revealed deficiencies in the manufacturing process. Although those deficiencies don't necessarily mean that the defibrillators on the market will harm patients, the FDA said it is requiring corrections to "ensure the continued availability of safe, effective and reliable products."
Copyright 2008 by United Press International