Early treatment of stomach infection may prevent cancer
Based on research using a new mouse model of gastritis and stomach cancer, researchers from the Massachusetts Institute of Technology (MIT) say that prompt treatment of Helicobacter pylori (H. pylori) infections reverses damage to the lining of the stomach that can lead to cancer.
In the May 1 issue of Cancer Research, a journal of the American Association for Cancer Research, researchers say their study results should lay to rest any question about whether – and when - antibiotic treatment of H. pylori can eliminate or reduce risk of developing gastric, or stomach cancer.
“We concluded that H. pylori eradication prevented gastric cancer to the greatest extent when antibiotics were given at an early point of infection, but that eradication therapy given at a later time point also delayed the development of severe lesions that can lead to cancer,” said the study’s lead author, James G. Fox, D.V.M., professor and director of the Division of Comparative Medicine at MIT.
The findings are important, Fox says, because stomach cancer is the second leading cause of cancer death worldwide, and approximately half of the world’s population is infected with H. pylori. Although H. pylori infection is now recognized as the major cause of both peptic ulcers and gastric cancer, and has been classified as a group I carcinogen by the World Health Organization, physicians are not sure whom to screen and treat with costly antibiotics, aside from first degree relatives of gastric cancer patients and those with peptic ulcer disease, he adds.
Since it typically takes several decades for gastric cancer to develop in those who are susceptible – which is estimated to be up to three percent of infected people – researchers also do not know when to treat the infection for maximum benefit. Human studies that tested treatment in patients who had already developed tumors had mixed results, but one previous study showed that giving antibiotics before premalignant lesions develop was successful in preventing cancer, Fox says.
The current study examined the effects of treating and eliminating H. pylori at different stages of progression from gastritis, an inflammation of the mucous membrane layer of the stomach, to development of gastric cancer. To do this, Fox and colleagues from MIT and Columbia University developed transgenic “INS-GAS” mice that over-expressed gastrin, a hormone that controls secretion of gastric acid by the stomach’s parietal cells.
“If you lose these cells over time, they stop secreting gastric acid, and this is, in and of itself, a risk factor for development of cancer, but gastric acid also helps protect against commensal bacterial colonization of the stomach,” Fox said.
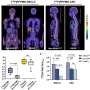
With increasing age, parietal cells in INS-GAS mice stopped producing gastric acid and underwent precancerous changes. By 20 months of age, the mice spontaneously developed invasive gastric cancer. Infection by H. pylori and progression to gastric cancer was accelerated in these mice, researchers discovered.
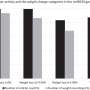
Researchers then treated the mice with antibiotics and looked for cellular changes. They found that, at every stage of advancing infection, mice that were treated with antibiotics had less severe disease. . Treating mice that were eight weeks post-infection reduced risk of developing cancer to the same level seen in uninfected mice. But using antibiotics at 12 and 22 weeks post-infection did not reverse the damaging changes, such as inflammation and development of precancerous lesions, to the levels seen in uninfected mice.
“Our mouse model mimics the progressive process we know occurs in development of human gastric cancer,” Fox said. “This shows early intervention provides the maximum benefit.”
Of added benefit, Fox says, is the associated finding that antibiotic treatment also reduces the level of other bacterial species that have invaded the stomach. “Gastric acid is a barrier to bacteria, and if there is no barrier, bacteria can move into the stomach from the lower bowel and colonize it, producing inflammation and progression to cancer,” he said. . “Findings in humans and mice now suggest that antibiotic treatment potentially changes gastric microbiota and may impact gastric carcinogenesis.”’
Source: American Association for Cancer Research