Study identifies mechanism underlying multidrug resistance in fungi
A team of researchers led by Anders Näär, PhD, of the Massachusetts General Hospital (MGH) Cancer Center has identified a mechanism controlling multidrug resistance in fungi. This discovery could help advance treatments for opportunistic fungal infections that frequently plague individuals with compromised immunity, such as patients receiving chemotherapy, transplant recipients treated with immunosuppressive drugs, and AIDS patients. The findings appear in the April 3 issue of Nature.
Almost 10 percent of bloodstream infections are caused by pathogenic fungi, such as the Candida species; and the mortality of such infections is approaching 40 percent. Just as many bacterial strains have become resistant to important antibiotics, resistance to common antifungal drugs is an increasing phenomenon in pathogenic fungi. To better understand the molecular pathways controlling multidrug resistance in fungi, the research team first investigated drug resistance in baker’s yeast, a common genetic model for observing biological processes.
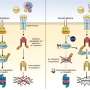
Using detailed genetic, biochemical, and molecular approaches, the researchers found that yeast induce multidrug resistance via a molecular switch similar to one that removes drugs and other foreign substances from human cells. When the yeast protein Pdr1p binds to antifungal drugs or other chemicals, it switches on molecular pumps that remove the drugs from the cell. The research team showed that this chemical switch also controls drug resistance in an important human pathogenic fungus, Candida glabrata. In humans, a protein called PXR is the drug sensor that turns on genes involved in detoxifying and removing drugs from cells.
“This intriguing similarity between the regulatory switches controlling multidrug resistance in fungi and drug detoxification in humans will allow us to take advantage of the extensive knowledge of the human molecular switch and identify new therapies for resistant fungal infections in patients with compromised immunity,” says Näär, an assistant professor of Cell Biology at Harvard Medical School (HMS).
The researchers also found exactly how Pdr1p turns on the multidrug resistance program. After binding to drugs, the Pdr1p protein partners with another key mediator of genetic switches called Gal11p. In-depth molecular and structural studies – in collaboration with the team of co-author Gerhard Wagner, PhD, Elkan Blout Professor of Biological Chemistry and Molecular Pharmacology at HMS – identified the specific area of Gal11p that binds to Pdr1p to induce multidrug resistance.
“This detailed understanding of the interaction between these proteins will allow screening for small-molecule inhibitors of protein binding. Such inhibitors may lead to novel co-therapeutics that will sensitize multidrug-resistant fungal infections to standard antifungal therapy,” says Wagner.
To further investigate the relevance of their findings, the researchers used a C. elegans roundworm model system – recently developed by co-author Eleftherios Mylonakis, MD, PhD, of MGH Infectious Disease – to study fungal pathogens. They found that worms infected with Candida glabrata that lacked either the Pdr1p or Gal11p proteins could be successfully treated with typical antifungal medications, suggesting that targeting the gene switch controlled by those proteins’ interaction could restore the effectiveness of standard drugs.
“Fungal infections have a serious impact on immunocompromised patients, and the development of resistance is particularly worrisome, since targets for antifungal drugs are limited,” says Mylonakis, an assistant professor of Medicine at HMS. “Given these concerns, having the opportunity to use our model system for the in vivo investigation of this resistance mechanism has been a particularly fulfilling endeavor.”
Source: Massachusetts General Hospital