Long-term muscle improvements shown in gene therapy study in mice
Injecting a gene responsible for making a specific protein into a mouse that’s used as a model for muscular dystrophy can lead to long-term improvements in the animal’s muscle size and strength, a new study shows.
Researchers investigating the gene delivery of the protein in animals suggest the results warrant testing the same approach in human clinical trials for diseases associated with muscle wasting, including Duchenne muscular dystrophy, the most common form of the childhood disorder.
Scientists used a safe virus to deliver a protein called follistatin into the leg muscles of young and older mice that have a disorder similar to human Duchenne muscular dystrophy (DMD). The protein inhibits the activity of myostatin, identified in previous research as a protein that limits muscle growth. Both young and old mice treated with the therapy responded with increased muscle mass and improvements in strength.
Because the older mice in the study responded well to the protein, the therapy might hold promise for older patients with DMD who have few treatment options once their muscles have experienced progressive degeneration, said principal investigator Brian Kaspar, an assistant professor of pediatrics at Ohio State University.
“These studies are significant given that there was functional effect on muscle enhancement even when treated at later stages in the mouse model,” Kaspar said.
The study also takes a rare long look at the effects of the therapy.
“Many studies don’t evaluate a therapy over a two-year time span. In our studies, the beneficial effects persisted over the two years we evaluated,” Kaspar said. “Furthermore, this long-term study shows that there were no obvious safety problems with either the gene therapy virus or the therapeutic protein, follistatin.”
The research is reported online in this week’s edition of Proceedings of the National Academy of Sciences.
DMD affects about one in 3,500 boys, who can show early symptoms of muscle degeneration and typically lose the ability to walk between age 6 and 12. With progressive disease, most patients die of respiratory failure or cardiac dysfunction in their 20s. Girls can carry the gene that causes the disease, but most have no symptoms.
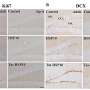
The mice used in this study as a model for DMD are called mdx mice. The older mdx mice received the therapy when they were 210 days old, at least a month after they showed significant hallmarks of their disease, including inflammation and fibrosis. When they were 560 days old, the treated mice showed robust muscles, with increased muscle fiber size along with reduced inflammation and less scarring compared to control-treated mdx mice.
In studies of younger mdx mice, the therapy was administered when they were 3 weeks old. At age 5 months, they had a larger body mass and higher muscle weight than did comparison animals.
Mice used for comparison were treated with an inactive fluorescent protein that allowed researchers to monitor which cells were affected by the experimental gene therapy technique.
Before testing follistatin in mdx mice, the scientists first tested the protein in normal mice and found after 725 days that they, too, had increased muscle mass and better grip strength when compared to untreated mice.
The resulting muscle enhancements in all of the treated mice were evident at the site of injection as well as in tricep muscles, meaning the therapy was able to affect other muscle cells in the body. To deliver the protein, scientists used an adeno-associated virus that had been manipulated to find its way into target cells without promoting any spread of the virus itself.
Previous research has shown that simply eliminating myostatin isn’t sufficient to improve muscle function in muscles of older DMD patients once progressive degeneration has begun. But the follistatin treatment in this latest study appeared to do more than just inhibit the effects of myostatin.
“This protein led to muscle enhancement, increased strength and lowered the effects of inflammation and fibrosis,” Kaspar said. “Because of those effects, we believe that it could be potentially useful for older Duchenne muscular dystrophy patients. And these results appear to translate to other muscle wasting diseases and aging, so it has potential to help a larger population of patients.”
The researchers selected a specific type of follistatin, known as the human FS-344 variant, in part for safety reasons, because some forms of the protein have been associated with disrupting reproductive function. After two years, the therapy in these studies showed no effects on reproductive function or cardiac function in the mice.
Kaspar is among researchers in the Center for Gene Therapy within the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, testing another kind of gene therapy in DMD patients in which the properties of the gene they are missing, dystrophin, are replaced.
“The muscle damage that older DMD patients have experienced may preclude them from having the gene correction performed. We think combination therapies may be another step in the research concerning older Duchenne patients, and follistatin could be part of that combination,” said Jerry Mendell, a co-author of these new studies, professor of pediatrics at Ohio State and director of the Center for Gene Therapy at Nationwide Children’s Hospital.
Source: Ohio State University