Two-way cell talk provides clues about neuromuscular disease
It’s a scientific given that neurons tell other cells what to do, but new evidence suggests that, like with any good relationship, these target cells also have much to contribute, scientists say.
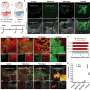
In an animal model, Medical College of Georgia researchers have shown that if a muscle cell fails to produce the protein beta-catenin, its neuron doesn’t develop or function properly.
Their finding provides some of the first proof that in vertebrates such as man, this retrograde communication – from the target cell back to the neuron – is essential, says Dr. Lin Mei, corresponding author on research published online Sept. 17 in Nature Neuroscience.
“Previously, we thought signals flow mainly from neuron to muscle. This shows they can be produced from muscle,” says Dr. Mei, MCG’s chief of developmental neurobiology and Georgia Research Alliance Eminent Scholar in Neuroscience. “This is some of the first clear genetic evidence that when you disturb something in the muscle, you have a nerve problem.”
Dr. Mei’s research team knocked out beta-catenin in the muscle cells of a developing mouse. As a result, nerve terminals, which reach out to target cells, were misaligned. Release of neurotransmitters, which enable cell talk, from the tiny vesicles inside nerve terminals was impaired. Mice died prematurely. “Two-way communication is absolutely essential,” he says. Interestingly when the researchers knocked beta-catenin out of neurons instead, neurons developed and functioned normally.
“Theoretically the finding is very important in that it supports the retrograde hypothesis,” Dr. Mei says. “Practically it is also important because problems with motor neuron survival and differentiation cause many neuromuscular diseases, such as muscular dystrophy and ALS, where motor neurons need to survive,” noting that it’s unknown why neurons die in these diseases.
“We believe there is a retrograde signal downstream of beta-catenin or regulated by beta- catenin,” says Dr. Mei. “If you don’t have beta-catenin in the muscle, that signal may be missing and motor neurons are not happy.”
To find out what that signal is, his lab is comparing genetic expression in the beta-catenin knockout mouse to that of a normal mouse to see which genes are up- or down-regulated. “Those genes may be targeted by beta-catenin and may serve as this retrograde signal. If we can identify that, I can retire,” says Dr. Mei.
Beta-catenin is a protein with many roles, including helping cells stick together, and regulating gene expression in the Wnt pathway, which is essential for development. Dr. Mei’s previous work has shown that at least in a Petri dish, when a signaling component of the Wnt pathway, called disheveled, is disturbed in muscle cells, it causes problems with their co-cultured neurons.
In the early 1900s, German-born Scientist Viktor Hamburger provided some of the first evidence of the importance of retrograde communication in proper development of motor neurons: when he removed the budding limbs of chick embryos, motor neurons decreased in number.
“...(T)he use of transgenic animals has established the importance of muscle ß-catenin in (neuromuscular junction) formation in vivo,” write Drs. Amy K.Y. Fu, Zelda Cheung and Nancy Y. Ip, of Hong Kong University of Science and Technology in an accompanying News and Views. “These findings also underscore the emerging role of Wnt signaling proteins in the regulation of synapse development. The identification of muscle ß-catenin-dependent signals for motoneurons may also contribute to our understanding of neuromuscular disorders, including muscular dystrophy and amyotrophic lateral sclerosis.”
Source: Medical College of Georgia