New chimeric mouse model for human liver diseases, drug testing
Cells cultured in the lab are like a fish out of water. Often, their behavior does not reflect their biological function within an entire organ or organism, which, for example, turns studying human liver cells into a big challenge.
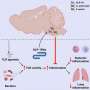
One way to get around the altered properties of the stranded cells is to populate mouse livers with human hepatocytes in the hope of creating a natural environment, which is exactly what researchers at the Salk Institute for Biological Studies did. They developed a simple system that allows them to transplant human hepatocytes into immunodeficient mice, which can now be used to test how drugs affect the liver.
“Rodents are often used as model organisms to study the efficacy and toxicity of drugs,” says lead author Karl-Dimiter Bissig, M.D. Ph.D., a postdoctoral researcher in the Laboratory of Genetics, “but mouse and rat hepatocytes may function in very different ways when it comes to metabolism of drugs.”
In the past, this has led to unexpected toxicity problems, when drugs moved into clinical trials after toxicity tests in rats failed to reveal adverse effects (e.g. Troglitazone). But it also worked the other way around. “The clinical introduction of furosamide, a powerful but perfectly safe diuretic, has been slowed down because of its hepatotoxicity in rats,” says Bissig.
The work, which will be published in this week’s online edition of the Proceedings of the National Academy of Sciences also holds promise for a better understanding of infectious diseases that affect the liver. “It is basically impossible to grow human hepatocytes in the lab, which was a big hurdle for the study of viruses such as hepatitis A and hepatitis B,” says senior author Inder Verma, Ph.D., a professor in the Laboratory of Genetics.
But most importantly, Bissig says, the mice will be an invaluable tool to advance regenerative medicine. “Many inherited disorders affecting liver metabolism could be cured if only five percent of all hepatocytes would express the missing enzyme,” he says.
In fact, that’s the underlying principle of the Salk researchers’ new chimeric mouse. It is based on a murine model for hereditary tyrosinaemia type I, developed by researchers at Oregon Healthy & Science University. An enzymatic defect in the tyrosine catabolism results in a toxic accumulation of byproducts within hepatocytes unless the mice are treated with a drug called NBTC.
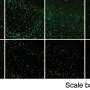
Withdrawing the drug allows to selectively expand hepatocytes that do not have this defect, such as transplanted human hepatocytes. Within three months of transplantation, up to 20 percent of the mouse liver is repopulated by human hepatocytes. But what’s more, the transplanted cells keep producing a foreign protein slipped inside with the help of a lentiviral vector, the kind usually used for gene therapy. “We are very excited about that aspect since very often cells shut off the production of proteins introduced as part of gene therapy,” says Verma.
Source: Salk Institute