MIT works toward novel therapeutic device
MIT and University of Rochester researchers report important advances toward a therapeutic device that has the potential to capture cells as they flow through the blood stream and treat them. Among other applications, such a device could zapp cancer cells spreading to other tissues, or signal stem cells to differentiate.
Their concept leverages cell rolling, a biological process that slows cells down as they flow through blood vessels. As the cells slow, they adhere to the vessel walls and roll, allowing them to sense signals from nearby tissues that may be calling them to work. Immune cells, for example, can be slowed and summoned to battle an infection.
“Through mimicking a process involved in many important physiological and pathological events, we envision a device that can be used to selectively provide signals to cells traveling through the bloodstream,” said Jeffrey M. Karp of the Harvard-MIT Division of Health Sciences and Technology. “This technology has applications in cancer and stem cell therapies and could be used for diagnostics of a number of diseases.”
The team, led by Karp, started with technology to induce cell rolling for research. With an implantable therapeutic device in mind, they improved that cell rolling technology to make it safe, more stable and longer lasting.
The improvements are described in the October 20 online issue of the journal Langmuir, published by the American Chemical Society.
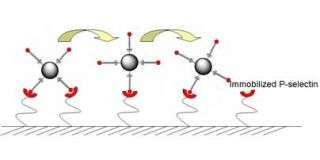
In the body, P-selectin and other selectin proteins regulate cell rolling in blood vessels. When P-selectin is present on a vessel's inner wall, cells that are sensitive to it will stick to that patch and begin to roll across it.
To induce rolling in the laboratory, the original technology weakly adheres P-selectin to a glass surface and flows cells across it. This surface treatment remains stable for several hours then breaks down. “While this method is useful for experiments, it's not good for long-term stability,” said Karp. An implantable device needs a coating that lasts weeks or even months so that patients won't need to come in frequently for replacements.
To improve the technology, the team experimented with several chemical methods to immobilize P-selectin on a glass surface. They identified a polyethelene glycol (PEG) coating that strongly bonded to P-selectin. This coating is also “non-fouling,” meaning it does not react with or accumulate other proteins, so it is potentially safe for use in an implant.
P-selectin remains stable on this coating for longer than the original technology. In tests with microspheres coated with a molecule that interacts with P-selectin, these spheres slowed down significantly as they flowed over the surface coated with layers of PEG and P-selectin. The effect was stable past four weeks, the longest the devices have been tested.
To validate that this technology works with cells that are sensitive to P-selectin, the team flowed neutrophils (white blood cells) across the coated surface. They too slowed and rolled on surfaces treated with the new coating, and the effect again lasted for at least four weeks.
The next step is translating these results to animal studies and using the technology to slow and capture stem cells and cancer cells circulating in the blood stream.
Ultimately CellTraffix, Inc., a sponsor of this technology and its licensee, wants to apply the technique to a device that is either implanted into the blood stream or appended as a shunt. In addition to PEG and selectin molecules, the device would also include a therapeutic agent. Such an agent would interact only with certain cells for a specific purpose.
According to University of Rochester biomedical engineering professor Michael King, who developed the concept for adhesive capture and reprogramming of cells, the device could, for example, slow down metastatic, or spreading, cancer cells and kill them.
Source: Massachusetts Institute of Technology