July 7, 2014 weblog
MicroCHIPS develops contraceptive implant
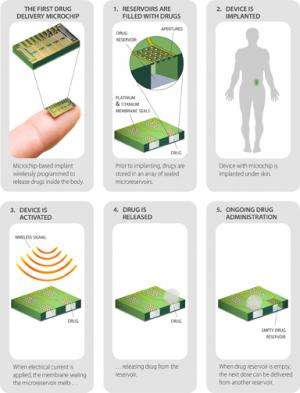
Scientists are making drug development news with their investigations into ways that enable implantable devices to deliver drugs, cutting the wear and care of traditionally delivered pills and injections. Now a Lexington, Massachusetts, drug delivery technology. company MicroCHIPS, has developed a contraceptive implant that can be deactivated and reactivated via wireless remote. The woman, with a remote control, could turn off the implant or with another click of the remote could restart it. According to a report about the device in MIT Technology Review, it measures 20 x 20 x 7 millimeters, implanted under the skin of the buttocks, upper arm, or abdomen. The implant is designed to last up to 16 years.
How does it work? The device is designed to dispense 30 micrograms a day of levonorgestrel. The latter is a progestin used as an ingredient in some hormonal contraceptives, Sixteen years' worth of the hormone fits in tiny reservoirs on a microchip 1.5 centimeters wide inside the device. MicroCHIPS has a hermetic titanium and platinum seal on the reservoirs containing the levonorgestrel. Passing an electric current through the seal from an internal battery melts it temporarily, allowing a small dose of the hormone out each day.
After 16 years, the device could be removed. Currently, said technology review, no hormonal birth control lasts over five years.
More work remains before MicroCHIPS files an application with the Food and Drug Administration. For example, said MIT Technology Review, it will be necessary to encrypt the chips to keep their wireless data flow private and secure. The device will begin pre-clinical testing next year in the U.S. The goal, said the report, is to have it on the market by 2018.
Relaying the history of the idea, MIT Technology Review said it originated two years ago when Bill Gates and colleagues visited Robert Langer's MIT lab. (The Langer Lab is an intersection between biotechnology and materials science.) Gates and his colleagues asked Langer if it were feasible to create birth control that a woman could turn on and off and use for many years. Langer thought the controlled release microchip technology he invented with colleagues Michael Cima and John Santini in the 1990s and licensed to MicroCHIPS might be key to a solution. The company's platform is based on the proprietary microreservoir technology that was first developed at MIT. This is a platform incorporating long-term implantable drug delivery technologies with wireless communications.
In February 2012, MicroCHIPS, announced results of a study published in the online edition of the journal Science Translational Medicine, in what the company said was the first successful human clinical trial with an implantable, wirelessly controlled and programmable microchip-based drug delivery device. In the trial, post menopausal women diagnosed with osteoporosis received daily doses of teriparatide through microchip delivery rather than daily injection. Seven patients between the ages of 65 and 70 received the microchip-based implant. The company announced the device and drug combination were found to be biocompatible with no adverse immune reaction. The microchip device was implanted and explanted using local anesthetic. MIT Technology Review reported that the device will begin pre-clinical testing next year in the U.S.
© 2014 Tech Xplore