Pig model of cystic fibrosis improves understanding of disease
It's been more than 20 years since scientists first discovered the gene that causes cystic fibrosis (CF), yet questions about how the mutated gene causes disease remain unanswered.
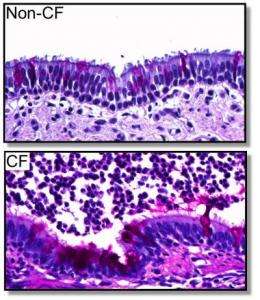
Using a newly created pig model that genetically replicates the most common form of cystic fibrosis, University of Iowa researchers have now shown that the CF protein is "misprocessed" in the pigs and does not end up in the correct cellular location. This glitch leads to disease symptoms, including gastrointestinal abnormalities and lung disease in the pigs, which mimic CF in humans. The findings are published in the March 16 issue of the journal Science Translational Medicine.
The findings match earlier laboratory experiments that suggested the gene mutation disrupts the process whereby the CF protein is folded into its correct shape and shipped to the membranes of cells that line the airways and other organs.
When it is correctly located at the cell membrane, this protein -- called cystic fibrosis transmembrane conductance regulator (CFTR) -- forms a channel to allow chloride ions to move in and out of cells. This ion movement is a critical component of the system that maintains salt and water balance across cell membranes in the lung as well as other organs and supports normal membrane function including eradicating bacteria from cell surfaces.
The new study shows that in pigs, the CFTR protein behaves the same way in a living animal as it does in experimental cell systems, suggesting that these experimental systems are useful for learning about the CFTR protein's properties. The cell systems and the new pig model may also be helpful in testing therapies designed to increase the amount of protein that gets to the cell membrane, or boost the activity of the protein that is located at the membrane.
"Instead of just trying to treat the symptoms of CF, current research is moving toward therapies that target mutations in the CFTR gene," said David Stoltz, M.D., Ph.D., UI assistant professor of internal medicine and senior study author. "For example, there already are drugs known as "correctors" being tested. These drugs help CFTR move from inside the cell to its correct location on the cell surface.
"The pig model could help us develop and test more corrector drugs, and it will also help us better understand why the protein is misprocessed in the first place," Stoltz added. "If we understand what is going wrong, we may be able to develop new therapies that can target the problem and allow more of the CFTR to make it to the cell surface, which may alleviate the disease symptoms."
In 2008, the UI team and colleagues at University of Missouri created pigs that were missing the CFTR protein. These animals developed CF disease symptoms that closely mimicked the human disease. In the new pig model, the animals have two copies of the CFTR gene containing the most common CF-causing mutation, which is known as the delta F508 mutation. These pigs also develop CF symptoms similar to the human disease. In particular, the CF pigs are born with gastrointestinal disease and develop lung disease over time.
By studying the protein in the pigs, the researchers were able to show that most of the CFTR protein is misprocessed and gets degraded, but a small amount of the protein does get to the cell membrane where it is able to form active chloride channels. However, the level of activity is only about 6 percent of the activity found in normal pigs with fully functional CFTR channels. The study shows that this small amount of CFTR activity is not sufficient to prevent CF disease in the pigs.
CF is a recessive disease, meaning a person with one mutated copy and one good copy of the CFTR gene is a "carrier" but does not have CF. This suggests that 50 percent of normal CFTR activity is sufficient for health. The question has always been, 'Is there a minimal amount of active CFTR that would be enough to protect people from the disease symptoms?'
"We know that people with 50 percent CFTR function have no disease, and now we know that 6 percent of full activity is not enough to prevent disease in the pigs," Stoltz said. "We still don't know how much CFTR is enough to prevent the disease, but this model animal could give us a way to investigate."


















