New anti-clotting drug added to recommendations for treating irregular heartbeat
The newly approved drug dabigatran is an alternative to warfarin to help prevent dangerous blood clots in patients with atrial fibrillation, according to updated guidelines from the American College of Cardiology, American Heart Association and the Heart Rhythm Society.
The "Focused Update" — published in Circulation: Journal of the American Heart Association, Journal of the American College of Cardiology and HeartRhythm Journal — specifically updates the section on emerging antithrombotic agents in atrial fibrillation treatment guidelines released by the three organizations on Dec. 20, 2010.
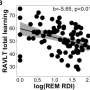
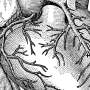
Atrial fibrillation is an irregular heart rhythm that occurs when the heart's two upper chambers beat erratically, causing the chambers to pump blood rapidly, unevenly and inefficiently. Blood can pool and clot in the chambers, increasing the risk of stroke or heart attack. More than two million Americans live with the condition.
According to this most recent update, dabigatran is useful as an alternative to warfarin to prevent stroke and blood clots in patients with either paroxysmal (recurrent episodes that stop after seven days) or permanent (an on-going episode) atrial fibrillation, and with risk factors for stroke or blood clotting who do not have a prosthetic heart valve, significant heart valve disease, severe renal failure or advanced liver disease.
Warfarin, an anti-clotting drug used since the 1950s, requires patients to have regular testing to monitor its effectiveness and dosage adjustment.
In December 2010 the atrial fibrillation guidelines were updated and recommended that a combination of aspirin and the oral antiplatelet drug clopidogrel might be considered to prevent stroke or other types of blood clots in patients with atrial fibrillation who are poor candidates for the clot-preventing drug warfarin.
More information: FDA medication guide for dabigatran – note important guidance on storing this medicine: www.fda.gov/downloads/Drugs/Dr … Safety/UCM231720.pdf