Manmade protein shows promise for cancer, macular degeneration

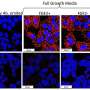
Potentially blinding blood vessel growth in the cornea resulting from eye injury or even surgery can be reduced by more than 50 percent with a new manmade protein, researchers say.
"We believe eventually we'll be able to use this protein to help patients in many situations where blood vessel formation is detrimental, including cancer, diabetic retinopathy and macular degeneration," says Dr. Balamurali K. Ambati, corneal specialist at the Medical College of Georgia and Augusta Veterans Affairs Medical Center. Dr. Ambati is corresponding author of the study published in the November issue of Investigative Ophthalmology & Visual Science.
The body can produce new blood vessels to promote healing after trauma, such as a corneal transplant, a significant corneal scratch from a contact lens or retinal oxygen deprivation caused by diabetes or aging. This natural response, called angiogenesis, becomes detrimental when new growth obstructs vision or when a tumor pirates the process to survive.
In an animal model, researchers used the protein they developed to reverse obstructive growth as long as one month after injury, says Dr. Ambati. That's a very long time after injury in a mouse's lifetime, indicating even well-established blood vessels are susceptible to intraceptor-mediated regression, he says.
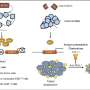
This intraceptor traps vascular endothelial growth factor, or VEGF, inside the protein making machinery of a cell. It's made with a portion of a VEGF receptor called sflt-1, a free-floating receptor recently shown to help keep the cornea clear by taking up and effectively neutralizing VEGF. Although other molecules have an anti-angiogenic effect, sflt-1 was the only one they found that spurs corneal blood vessels when blocked. The work, published in October in Nature, was led by teams at MCG and the University of Kentucky.
"Now we have designed a novel recombinant molecule where we take a subunit of sflt-1 and couple it with a four-amino-acid peptide tail," he says. "The tail essentially handcuffs the manmade molecule within the protein-making machinery of the cell so that it stays there and anything that binds with it, namely VEGF, stays there too. So it's a very specific way of down-regulating a target protein."
In May 2005, Dr. Ambati and his colleagues published work in Investigative Ophthalmology & Visual Science showing the intraceptor helped reduce blood vessel development in the test tube and animal models for corneal injury and melanoma.
"Now we are talking about making them go away," says Dr. Ambati. While the work is still in the laboratory, it provides further evidence of the intraceptor's potential clinical application, he says.
The work shows the intraceptor prompts regression of blood vessels by inducing programmed cell death, or apoptosis, in the vascular endothelial cells that line the vessels.
"The biology of all this is showing this molecule interrupts the proper folding of proteins involved in existing blood vessels, which makes them die. It's a nice result," says Dr. Ambati.
Some existing anti-angiogenesis treatments target VEGF outside cells. "It is important to bind it within cells because certain cells, such as cancer and blood vessel cells, have the capability to produce their own VEGF and their own receptors," Dr. Ambati says. "Imagine trying to block from the outside a factory that has everything it needs inside. You have to throw a monkey wrench inside the factory and that is what we managed to do."
For the study, the manmade protein was injected directly into the cornea with a microneedle. "Ideally we would like to develop a topical eye drop with a long-term delivery system," says Dr. Ambati.
His research team is pursuing its work of the intraceptor's potential role in destroying blood vessels that help sustain cancers. They also are looking at a biodegradable polymer cage so they can encapsulate the intraceptor, tag it with a homing device for target cells and deliver it "like a missile carrying a payload" into the desired cells where it will slowly release the intraceptor, he says.
Source: Medical College of Georgia