RUDN University chemist found a catalyst to improve biofuel production
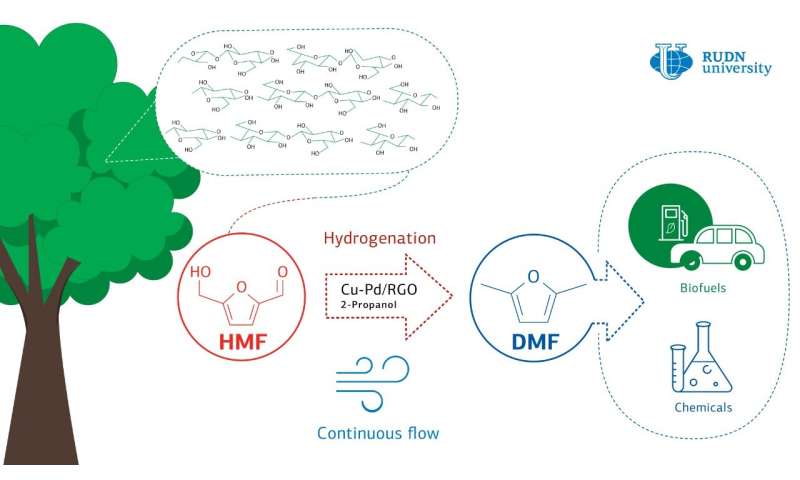
Chemists from RUDN University, Chulalongkorn University (Thailand), and the University of Cordoba (Spain) have developed a new method for the synthesis of biofuels from renewable vegetable raw materials. They proposed to use bimetallic catalysts for the production of 2,5-dimethylfuran (DMF) biofuel. The chemists managed to increase the yield of DMF by one third, up to 95%, and to prolong the stability of the new catalyst to 8 hours during a continuous chemical process of DMF production. The discovery will help improve biofuel production. The article was published in the journal ACS Sustainable Chemistry & Engineering.
DMF has a high energy density (30 kJ/cm3) and a high Research Octane Number (RON = 119), which makes it comparable in efficiency with gasoline. Research and production of DMF is carried out in Spain, China, Latvia, Russia, the USA, and Japan. Lignocellulose is used as raw material for the production of this biofuel; it is the most common biomass on the planet, based on grass, trees, and agricultural industry waste. But the production of DMF requires sophisticated equipment, pressures of up to 140 atmospheres, and rare chemical reagents. The biomass is transformed by dehydration reaction (the loss of water from molecules of organic compounds) of 5-hydroxymethyl furfural (GMF), after which the GMF is transformed into DMF by selective hydrogenation. However, undesirable by-products are also formed in the process, beside DMF itself, and the DMF yield does not exceed 60%. A team of chemists from Russia, Spain, and Thailand has studied several types of metal catalysts that might solve these problems.
Rafael Luque of the Research Institute of Chemistry (RIC) of RUDN University and his colleagues gave priority to a catalyst based on noble metals, which serve as the main active elements of catalysis. This helped avoid the formation of by-products and non-selective hydrogenation (the reaction of the addition of hydrogen atoms to molecules of organic matter). Propanol-2 was used as the hydrogen donor for the selective reduction of DMF.
Palladium was chosen, initially; however, a palladium-based catalyst was too expensive. Furthermore, the use of a palladium catalyst led to a decreased yield of DMF due to the fact that it did not interact with propanol-2 selectively enough. The solution was found in creation of a bimetallic catalyst based on palladium and inexpensive copper.
A bimetallic catalyst with 1-2% palladium content increased not only the yield of DMF, but also the stability of the reaction as compared to single metal catalysts. The palladium/copper interaction in the bimetallic catalyst increased the rate of reaction and prevented aggregation and leaching of active copper. The deactivation of the catalyst (the time before drop in performance due to a decrease in the yield of reaction products) was thus minimised. It appears that full conversion of GMF (processing) and DMF yield of up to 99% were achieved due to the smaller crystal size in the synthesised bimetallic catalyst.
"Reaction conditions were also changed, from cyclic to continuous. There were several advantages: faster and easier scaling, more control over setting of conditions for maximising and fine tuning the activity and selectivity with respect to the target products."
Under optimal conditions, in the course of the selective hydrogenation of GMF to DMF, the copper-palladium catalyst (10Cu-1Pd/RGO) demonstrated high stability for 8 hours during continuous chemical reaction, at a temperature of 180° C, a pressure of 15 bar, and a flow rate of raw materials 0.2 ml/min-1.
"This technology will increase the efficiency of biofuel production. While maintaining the volume of raw materials, the yield of finished products will increase, and the amount of waste will be at a minimum."
More information:
Sareena Mhadmhan et al. Continuous Flow Selective Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran Using Highly Active and Stable Cu–Pd/Reduced Graphene Oxide, ACS Sustainable Chemistry & Engineering (2019). DOI: 10.1021/acssuschemeng.9b03017
Provided by RUDN University