This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Building blocks for the future: Rhodium-catalyzed [2+2+1] cycloaddition achieves high enantioselectivity
![The proposed reaction provides a versatile route to valuable cyclic compounds for drug discovery and other applications. Credit: Tokyo Institute of Technology Building blocks for the future: enantioselective [2+2+1] cycloaddition reactions with rhodium catalysis](https://scx1.b-cdn.net/csz/news/800a/2024/building-blocks-for-th.jpg)
Cycloaddition reactions are an efficient strategy for constructing cyclic compounds that are important building blocks for other chemicals. In these processes, π-electrons from different unsaturated molecules, such as alkenes, alkynes, or dienes accommodate to form new cyclic structures in a single step. π-electrons are those responsible for the π-bonds in double and triple bonds. In a double bond, one of the C-C bonds is a π-bond, while in a triple bond, two of the C-C bonds are π-bonds.
During these reactions, the π-electrons rearrange to form new σ-bonds, resulting in the formation of ring structures. For example, in a [2+2+2] cycloaddition, three components each with two π-electrons combine to form a six-membered ring.
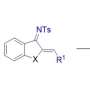
In a [2+2+1] cycloaddition, two components with two π-electrons each, along with an additional component acting as a single carbon unit, combine to form a five-membered ring. These reactions are highly efficient because most of the reactants are converted into the final product. While [2+2+2] reactions are well-established, producing compounds with the desired chirality using [2+2+1] reactions is challenging.
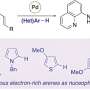
In a study published in the journal Nature Synthesis, a research team led by Professor Ken Tanaka from Tokyo Institute of Technology developed a rhodium (Rh)-catalyzed enantioselective [2+2+1] cycloaddition reaction. The reaction uses three different 2π-components—cycloalkenes, acetylenecarboxylates, and terminal alkynes—to produce chiral 3-methylenecyclopent-1-ene derivatives with exceptional selectivity.
"The ultimate goal for intermolecular cycloaddition is the catalytic selectivity control using three different 2π-components," says Tanaka. "As a catalytic version, we developed the sole example of the enantioselective intermolecular cross-[2+2+1] cycloaddition."
![The [2+2+2] and [2+2+1] cycloadditions of three 2π-components. Credit: Nature Synthesis (2024). DOI: 10.1038/s44160-024-00604-7 Building blocks for the future: Enantioselective [2+2+1] cycloaddition reactions with rhodium catalysis](https://scx1.b-cdn.net/csz/news/800a/2024/building-blocks-for-th-1.jpg)
Studies on the reaction mechanism showed that it begins with the coordination of cycloalkenes and acetylenedicarboxylates to the cationic Rh(I) catalyst, forming a rhodacyclopentene intermediate. The terminal alkyne then reacts with this intermediate to generate a vinylidene intermediate. Following this, a reductive elimination step produces the final cycloadduct while regenerating the Rh(I) catalyst.
One of the major advantages of this method is its compatibility with a diverse range of substrates. The researchers used oxabenzonorbornadiene and norbornene derivatives as cycloalkenes, di-tert-butyl and dimethyl acetylenedicarboxylates, and terminal alkynes like 1-dodecyne, phenylacetylene, and silylacetylenes. With phosphine ligands like (R)-BINAP and (R)-Segphos, they could carefully control the enantioselectivity and yield, achieving E isomers of 3-methylenecyclopent-1-ene derivatives with enantiomeric excess values ranging from 94% to over 99%.
The 3-methylenecyclopent-1-ene derivatives are incredibly useful building blocks for creating many different types of molecules. For instance, by adding hydrogen to these molecules (hydrogenation), the researchers produced multicyclic cyclopentenes. Epoxidation produced multicyclic epoxides, which could be further converted into a variety of hydroperoxides, alcohols and aldehydes.
With its vast potential to produce a diverse range of 3D compounds which are otherwise challenging to synthesize, this catalytic approach paves the way for producing novel compounds with diverse applications.
"The present [2+2+1] cycloaddition and subsequent transformations provide access to otherwise inaccessible three-dimensional (3D) compounds that are attractive for drug discovery research," says Tanaka.
More information: Kaito Shibahara et al, Rh-catalysed enantioselective [2+2+1] cycloaddition reactions using three different 2π-components, Nature Synthesis (2024). DOI: 10.1038/s44160-024-00604-7
Journal information: Nature Synthesis
Provided by Tokyo Institute of Technology