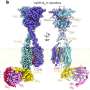
In a new study, researchers from Japan have developed a novel hybrid protein complex by binding lysozyme to copper, which has enhanced superoxide dismutase activity that can remove ROS more effectively. These complexes could find use in therapeutic drug applications. Credit: Daisuke Nakane and Takashiro Akitsu from Tokyo University of Science
In aerobic organisms, reactive oxygen species (ROS), such as hydroxide (OH), singlet oxygen (1O2), hydrogen peroxide (H2O2), and superoxide (O2–) ions are produced during aerobic respiration, which causes serious oxidative damage to biomolecules in the body. Hence, the removal of ROS, particularly O2–, is of primary importance as it reacts with H+ to produce other toxic ROS species like H2O2 and OH.
This is achieved by metalloenzymes called superoxide dismutases (SODs). These enzymes possess metal ions (like Ni, Fe, Mn, Cu, and Zn) in their active centers that catalyze the decomposition of O2– to H2O2 and O2. In this regard, low molecular weight Cu(II) complexes have gained importance as functional SOD models that exhibit high SOD activity. However, they are limited by their tendency to become toxic to the biomolecules after the release of Cu(II).
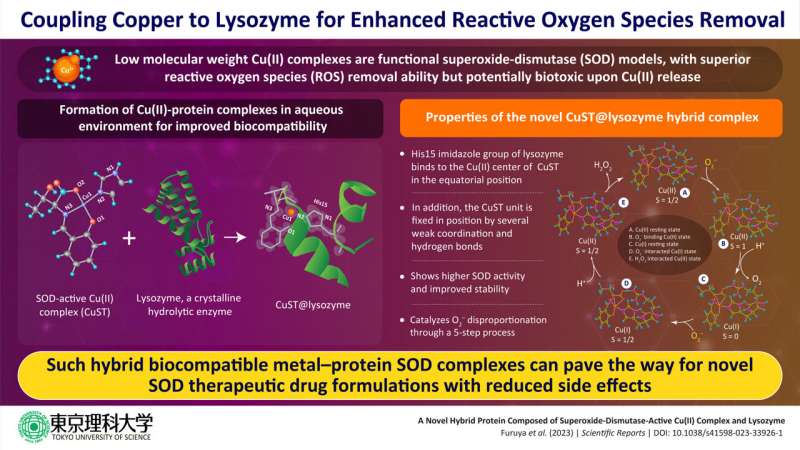
In a recent study, a group of researchers led by Assistant Professor Daisuke Nakane and Professor Takashiro Akitsu from the Department of Chemistry, Tokyo University of Science (TUS), has developed a novel metal-protein hybrid complex with enhanced ROS activity. They coupled the hydrolytic enzyme lysozyme with SOD-active Cu(II) complex to form the hybrid lysozyme CuST@lysozyme, which showed promising SOD activity but low biotoxicity.
"We investigated the formation of a hybrid protein composed of lysozyme and a functional SOD-mimetic Cu(II) complex. We chose lysozyme owing to its stability and crystallinity. We theorized that the resulting SOD-mimetic hybrid protein would improve the biocompatibility and stability of the functional SOD model Cu(II) complex," explains Dr. Nakane as the rationale behind their study. The study was published on in Scientific Reports.
Through detailed crystallographic and spectroscopic analysis, the research team, which also comprised Assistant Professor Kenichi Kitanishi from TUS, Dr. Arshak Tsaturyan from Southern Federal University, and Professor Masaki Unno from Ibaraki University, among others, confirmed the formation of the hybrid protein CuST@lysozyme, and elucidated its structure.
They report that the His15 imidazole group of the lysozyme binds to the Cu(II) center of CuST in the equatorial position while the CuST unit is fixed axially by several weak coordination and hydrogen bonds. Further they also suggest that O2– can coordinate to the Cu(II) center. Through assays, the researchers established high SOD activity and stability of the biocompatible CuST@lysozyme hybrid protein complex.
Based on their spectroscopic and quantum calculations, the team propose a five-step mechanism of O2– disproportionation by the complex. These steps are (1) Cu(II) resting state, (2) O2–-binding Cu(II) state, (3) Cu(I) resting state after protonation of the carboxylate ligand, (4) O2–-interacted Cu(I) state, and (5) H2O2-interacted Cu(II) state.
They further suggest that the stability of the complex can be improved by suppressing ligand dissociation by using late-transition-metal complexes for binding lysozyme, increasing interaction between the complexes and lysozyme by using ligands with hydrogen-bonding moieties, and introducing acidic functional groups to counter the basic side chains of lysozyme.
The study introduces a new class of SOD active hybrid protein complexes that are biocompatible and have no side reactions with bodily fluids after decomposition of the mimetic complex. "We have strategically improved the stability of the metal–lysozyme composites, specifically in biological fluids such as plasma and cytosol. This should pave the way for deeper discussions on their therapeutic applications," concludes Prof. Akitsu.
More information: Tetsundo Furuya et al, A novel hybrid protein composed of superoxide-dismutase-active Cu(II) complex and lysozyme, Scientific Reports (2023). DOI: 10.1038/s41598-023-33926-1
Journal information: Scientific Reports
Provided by Tokyo University of Science