This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study unlocks key information on major drug target for psychiatric and cognitive disorders, including schizophrenia
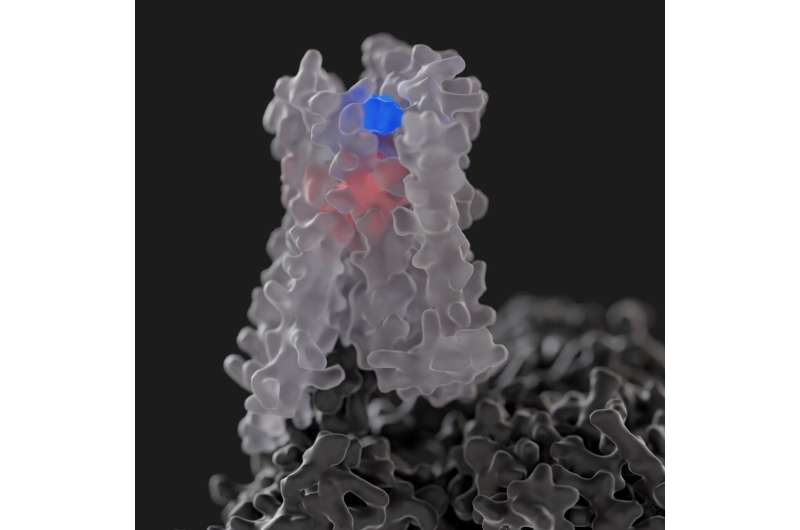
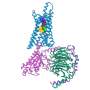
An international study led by Monash University has, for the first time, harnessed cutting-edge technology to reveal new and unprecedented information of how a special class of drugs, known as "allosteric modulators" work at the M4 muscarinic receptor, a major target for notoriously difficult-to-treat psychiatric disorders associated with cognitive deficits, such as schizophrenia.
Muscarinic receptors are members of the superfamily of G protein-coupled receptors (GPCRs)—the largest drug target class encoded by the human genome. The M4 muscarinic receptor subtype, in particular, plays a vital role in regulating neurotransmitters, such as dopamine and glutamate, in key areas of the brain involved in psychosis and cognition, but, until recently, was notoriously difficult to selectively target by conventional agonists or antagonists.
This challenge has begun to be addressed in recent years with the discovery of highly selective allosteric modulators for this receptor, but how such drugs achieve their selectivity and regulate M4 receptor activity in response to different activators has remained a major, elusive, knowledge gap, despite high clinical implications.
The new study, published in eLife, and led by a team of researchers from the Monash Institute of Pharmaceutical Sciences (MIPS), has now addressed this important gap in our molecular-level understanding of how allosteric modulators mediate their effects at the M4 muscarinic receptor.
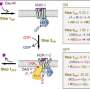
In this study, the MIPS researchers (who are world-leaders in the discovery and development of next-generation neuromedicines, including allosteric modulators, for difficult-to-treat mental illnesses), rigorously quantified the pharmacological properties of two distinct 'positive' allosteric modulators, alone or in combination with two different agonists, at the human M4 muscarinic receptor.
The team also tapped into technological advancements in the fields of cryo-electron microscopy (cryo-EM) and molecular dynamics simulations to unlock first-of-its-kind evidence of key molecular mechanisms underlying allosteric pharmacology, advancing our understanding of the structural basis of allosteric modulation of the M4 muscarinic receptor and paving the way for the discovery and design of future allosteric drugs.
In particular, the researchers revealed a vital role for ligand–receptor–G protein complex stability, mediated by conformational dynamics between these sites, in the ultimate determination of affinity, efficacy, degree of allosteric modulation, and mechanisms underlying species variability of allosteric drugs, all of which have significant implications for preclinical-to-clinical discovery and translation of such agents.
Corresponding author, and MIPS Senior Research Fellow, Dr. David Thal, said that allosteric modulation of GPCRs is a major paradigm in drug discovery; however, despite decades of research, a molecular-level understanding of the general principles that govern the myriad pharmacological effects exerted by GPCR allosteric modulators has remained elusive.
"By determining the cryo-EM structures of the M4 muscarinic receptor and applying molecular dynamics simulations, the team has provided a holistic framework for further GPCR mechanistic studies and, in turn, opened up new approaches for GPCR drug discovery," said Dr. Thal.
Professor Arthur Christopoulos, FAA FAHMS, also a corresponding author on the study, Dean of the Faculty of Pharmacy and Pharmaceutical Sciences, and Director of Monash's Neuromedicines Discovery Centre, said the results have broader implications for the development of new allosteric medicines designed to reduce currently untreated symptoms associated with schizophrenia and cognitive disorders.
"The vast majority of patients with schizophrenia have impaired cognition, which is untreated by any existing antipsychotic drug, and its severity remains a major predictor of prognosis. However the development of new safe and effective treatments has remained stagnant for decades," said Professor Christopoulos.
"The research conducted in this study is a vital step toward progressing the next generation of medical therapies and innovations that could make a true impact when it comes to improving health and saving lives."
Dr. Celine Valant, also a corresponding author and Senior Research Fellow at MIPS, said the findings open up exciting new opportunities.
"The evidence presented in this study will enable future GPCR drug discovery research and potentially lead to the development of next generation medicines for the treatment of a range of psychiatric and cognitive disorders," said Dr. Valant.
More information: Ziva Vuckovic et al, Pharmacological hallmarks of allostery at the M4 muscarinic receptor elucidated through structure and dynamics, eLife (2023). DOI: 10.7554/eLife.83477
Journal information: eLife
Provided by Monash University