High-temperature shock (hts) features ultrafast heating/cooling rate (>105 k/s) and is suitable for the fabrication of catalytic high-entropy alloys (HEA). We present the physicochemical principles of hts, introduce three typical methods (joule heating, laser heating, and microwave heating), and review the progress of hea prepared by HTS. Credit: Chinese Journal of Catalysis
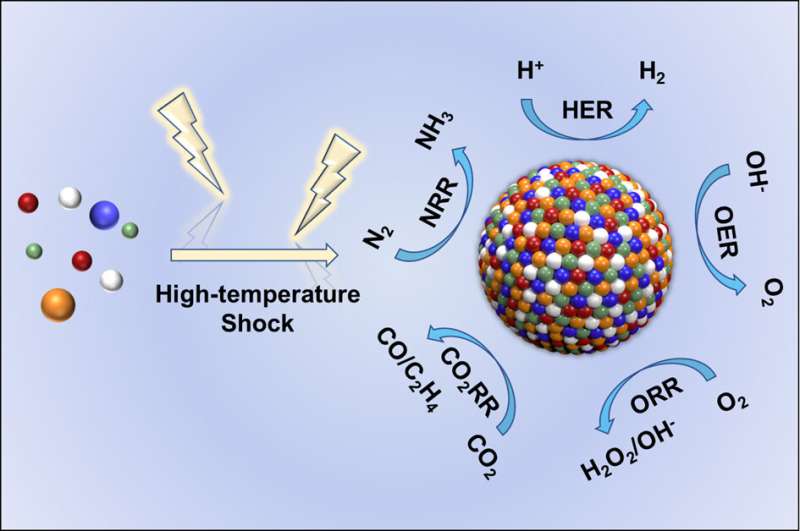
High-temperature shock (HTS) is an emerging synthesis method with kinetics-dominated non-equilibrium characteristics, which can achieve an ultrafast heating/cooling rate of ~105 K/s and a peak temperature larger than ~3000 K within a time scale of seconds or milliseconds, and is widely used in the preparation of high entropy content, thermodynamic metastable phase and defect-rich materials.
Among these significant advances, nanoscale high entropy alloys (HEA) are particularly prominent in heterogeneous catalytic reactions with remarkable activity, selectivity, and stability due to flexible composition space and high-entropy mixing structure. There are numerous reports on using the HTS technologies to fabricate HEA and other high entropy materials in recent years; however, the construction strategies and inherent physicochemical fundamentals of HTS still remain to be thoroughly summarized and explored.
Recently, a research team led by Prof. Yanan Chen from Tianjin University and Dr. Ye-Chuang Han from Xiamen University, China, presented physicochemical principles of HTS from the "energy-space-time" perspective, introduced representative HTS technologies (e.g., Joule heating, laser heating, microwave heating), summarized the advantages of HTS compared to traditionally thermodynamic-dominated near-equilibrium heating methods (e.g., tube furnace and muffle furnace) on synthesizing catalysts.
In addition, the concepts and features of HEA were introduced, and the latest progress in the synthesis of HEAs using the HTS techniques was reviewed. Finally, conclusions and perspectives were also provided for future investigations of HTS and HEA, which has great significance to guide both the theoretical and experimental development of HTS and its application in HEA controllable preparations. The results were published in Chinese Journal of Catalysis.
From the "energy-space-time" perspective, combining the law of conservation of energy and the relationship between heat and temperature, the factors of the heating rate for HTS can be deduced as follows: the absorption coefficient (ξ), the power of input energy (P), and the natural features (specific heat capacity (c), mass (m)) of materials.
The absorption coefficient is especially interrelated with the features of reactants and substrates, reaction environment, and heating approaches. In addition, the input energy directly effects on the target during HTS, therefore, the reaction volume (V) is a crucial parameter, and the heating rate depends on power density(E/t/V), which is the coupling of energy (E), space (V) and time (t).
The higher the power density (means the greater energy, smaller space, shorter time), the faster the heating rate. The cooling process is mainly determined by the natural features of the materials due to the lack of energy input.
HEA exhibits excellent catalytic activity and stability due to their four unique effects: thermodynamic high entropy, structural lattice distortion, kinetic sluggish diffusion, and "cocktail" effects. However, traditional methods used to prepare HEA have mostly resulted in large-sized bulk materials with small specific surface areas, thereby greatly hindering catalytic applications.
HTS can inhibit the coalescence and growth of HEA nanoparticles. The authors summarized the latest progress in the preparation of HEA and other high entropy materials using typical HTS technologies (Joule heating, laser heating, and microwave heating), as well as their applications in catalytic reactions.
Finally, outlooks were provided from the extreme non-equilibrium conditions and equipment of HTS, the rational design, advanced characterization and high-throughput screening of HEA.
More information: Yanchang Liu et al, High-temperature shock synthesis of high-entropy-alloy nanoparticles for catalysis, Chinese Journal of Catalysis (2023). DOI: 10.1016/S1872-2067(23)64428-6
Provided by Chinese Academy of Sciences