This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel crystal compound melts under ultraviolet light
While many materials melt when heated, researchers from Japan recently discovered a novel material in which melting can be induced by ultraviolet light instead of heat. Even more intriguing, this material exhibits changes in its luminescent properties while it melts. This material is the first organic crystalline material found to show changes in luminescent color and intensity upon ultraviolet light-induced melting.
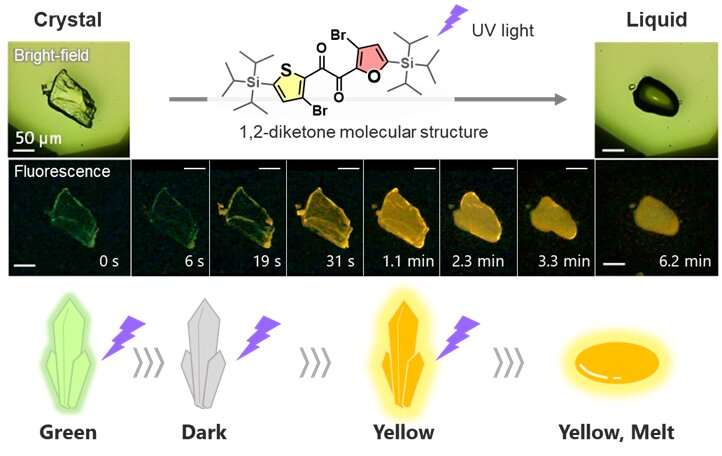
Investigators from Osaka University reported their discovery of this new class of photo-responsive crystal compounds, "heteroaromatic 1,2-diketones," in Chemical Science on April 20. Light irradiation causes the crystals in these materials to melt, a phenomenon termed photo-induced crystal-to-liquid transition (PCLT).
This phenomenon can dramatically change a material's properties and makes possible a broad range of applications, for example, photo-responsive, reversible adhesives that could be controlled by light. Few materials have been shown to have this crystal-melting property; hence, the discovery of a new class of PCLT materials is a great step forward in this field.
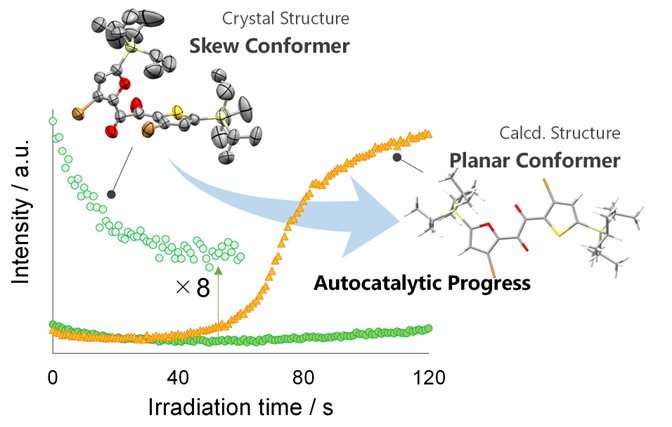
In characterizing their newly discovered class of PCLT materials, the researchers found that one member of this class, the diketone SO, shows changes in luminescence during the irradiation-induced melting process. "This is the first organic crystal we know of that exhibits a luminescent evolution during crystal melting, showing changes in intensity and color, from green to yellow," says lead author Mao Komura.
These changes in luminescence, i.e., changes in the way the material absorbs and emits light, indicated that SO was undergoing molecular-level changes in shape during the PCLT process. Building upon past research on luminescent molecules, the research team realized they could further investigate these molecular-level changes underlying PCLT to better understand the crystal-melting phenomenon.
"We found that the changes in luminescence arise from sequential processes of crystal loosening and conformational changes prior to melting," explains senior author Yosuke Tani. "These visual indications of the steps of the PCLT process enabled us to advance the current understanding of crystal melting at the molecular level."
By applying single-crystal X-ray analysis, thermodynamic property analysis, and theoretical calculations to probe the mechanisms governing the behavior of this new PCLT material, the researchers demonstrated that a disordered layer in the crystal is a key factor for PCLT in this class of materials.
This discovery of a novel PCLT material, along with its characterization, provides fundamental insights into the mechanism of crystal melting and will enable greater opportunities for designing PCLT materials with a variety of applications, including photolithography, thermal energy storage, and light-induced adhesion.
More information: Mao Komura et al, Photoinduced crystal melting with luminescence evolution based on conformational isomerisation, Chemical Science (2023). DOI: 10.1039/D3SC00838J
Journal information: Chemical Science
Provided by Osaka University