This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Humans vs. bacteria: Differences in ribosome decoding revealed
Scientists at St. Jude Children's Research Hospital revealed that human ribosomes decode messenger RNA (mRNA) 10 times slower than bacterial ribosomes, but do so more accurately. The study, published today in Nature, used a combination of field-leading structural biology approaches to better understand how ribosomes work. The scientists pinpointed where the process slows down in humans, which will be useful information for developing new therapeutics for cancer and infections.
Ribosomes are molecular machines within cells, responsible for synthesizing proteins by decoding mRNA. By conducting mechanistic studies on bacterial and human ribosomes, researchers can understand their similarities and differences to develop drugs and understand disease.
Many antibiotics, the drugs we use to treat bacterial infections, work by targeting bacterial ribosomes. In humans, changes in how accurately ribosomes decode mRNA have been linked to aging and disease, representing a potential point of therapeutic intervention. This gives the work implications for the treatment of infections and cancer.
"Bacteria have been very well studied for many decades, but the kind of studies that we do, careful mechanistic studies, have been missing on human ribosomes," said corresponding author Scott Blanchard, Ph.D., St. Jude Department of Structural Biology. "We're very interested in human ribosomes because those are what need to be targeted to find new treatments for cancer and viral infections such as COVID."
Resolution revolution
Ribosomes decode mRNA using a molecule called aminoacyl-transfer RNA (tRNA) as substrate. The decoding process involves several different steps.
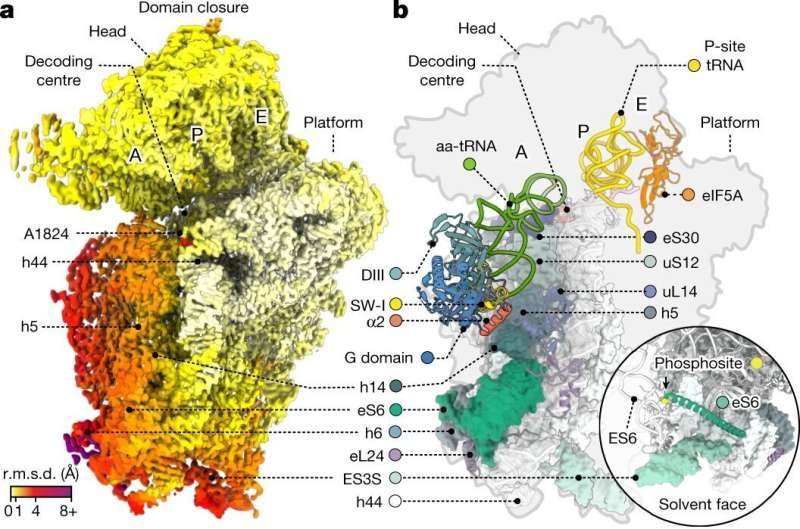
The researchers deployed methods such as single-molecule fluorescence resonance energy transfer (smFRET) and cryo-electron microscopy (cryo-EM) to examine the human ribosome decoding mechanisms. The single-molecule imaging gives the researchers information on how quickly things occur.
So, in this case, how quickly human ribosomes go through the different steps during the decoding process. Cryo-EM gives the researchers structural information. So, how the human ribosome looks or what conformations (shapes) it is in at each step. By combining these two methods, the scientists get information on how quickly these processes occur in humans compared to bacteria as well as about the underlying structural causes for any differences they observe.
"We wanted to know how quickly a human ribosome can read the genetic code, how quickly it finds the tRNA that's complementary to the mRNA," said co-first author Mikael Holm, Ph.D., St. Jude Department of Structural Biology. "We found that the process is about 10 times slower for human ribosomes than it is in bacteria. But this slow down adds accuracy, because human ribosomes are known to be more accurate at translating the code than bacterial ribosomes."
Specifically, the researchers found that while humans and bacteria both decode mRNA, the reaction pathway of aminoacyl-tRNA movement during the decoding process is different on human ribosomes and is significantly slower.
These differences arise from structural elements in the human ribosome and in the human elongation factor, eEF1A, that together are responsible for faithfully incorporating the right tRNA for each mRNA codon (piece of the sequence). The distinct nature and timing of conformational changes within the ribosome and eEF1A may explain how human ribosomes achieve greater decoding accuracy.
"With our cryo-EM structural studies, we were able to resolve human ribosome structures to atomic resolution, which revealed unprecedented features such as rRNA and protein modifications, ions and solvent molecules present in the human ribosome," said co-first author Kundhavai Natchiar, Ph.D., St. Jude Department of Structural Biology.
"These features finely characterize the molecular basis of interactions of the drug molecules with the human ribosome, which is indispensable for human ribosome–based drug design and discovery."
Caught in the act
The researchers also pinpointed exactly which step of the decoding process slowed down in human ribosomes. There are two steps in the process of the ribosome selecting the right tRNA: initial selection and proofreading selection. The second step, proofreading selection, is where the ribosome checks for a second time that it chose the right molecule. That is the step that is 10 times slower in humans than in bacteria.
Think of a gymnast, contorting themselves into different shapes on the mat as they work through their routine. This is similar to how ribosomes transition into various conformations to achieve different results. The research showed that a lot of conformational gymnastics that human ribosomes undergo are not present in bacterial ribosomes and are thus likely tied to the slowdown of the proofreading selection process.
The researchers also found that several drugs target the proofreading selection process, not initial selection. So, instead of hitting the step that is similar between humans and bacteria, these drugs focus on the most different, slowest step.
"In structural biology, a single snapshot of a macromolecular machine is not always sufficient to explain how it functions," said co-first author Emily Rundlet, Ph.D., St. Jude Department of Structural Biology.
"Often, the snapshot that's needed to answer your biological question is not the most stable form of the molecule, but instead it is short-lived and difficult to capture. Using smFRET and cryo-EM together brings the dimension of time to structural biology, which allows us to visualize important transient intermediate steps of human decoding and understand the different mechanisms on a new level."
More information: Mikael Holm et al, mRNA decoding in human is kinetically and structurally distinct from bacteria, Nature (2023). DOI: 10.1038/s41586-023-05908-w
Journal information: Nature
Provided by St. Jude Children's Research Hospital