Credit: Tokyo Tech
Oxides of tin (SnxOy) are found in many of modern technologies due to their versatile nature. The multivalent oxidation states of tin—Sn2+ and Sn4+—impart tin oxides with electroconductivity, photocatalysis, and various functional properties. For the photocatalysis application of tin oxides, a narrow bandgap for visible-light absorption is indispensable to utilize a wide range of solar energy. Hence, the discovery of new SnxOy could help improve the efficiency of many environmentally significant photocatalytic reactions like water splitting and CO2 reduction. While there are many theoretical and computational predictions of new stable SnxOy, there still remains a need for experimental studies that can turn the predictions into reality.
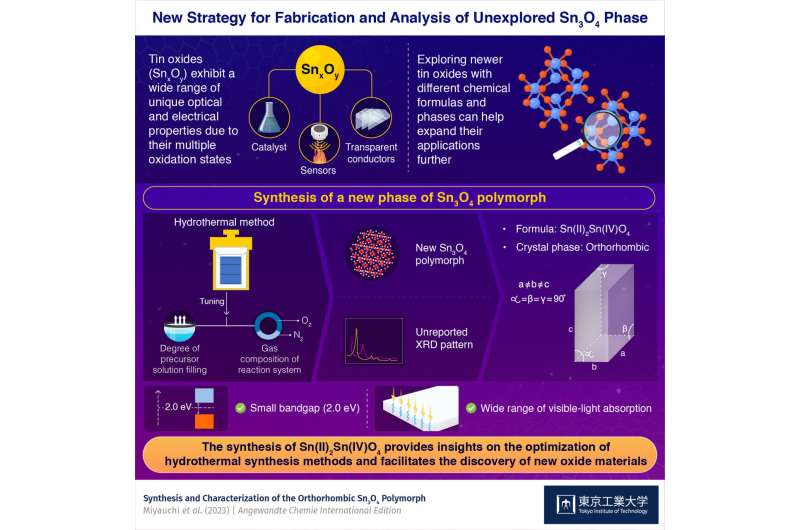
Taking this as a call to action, researchers from Tokyo Institute of Technology, National Defense Academy, and Mitsubishi Materials Corporation have designed a new tin oxide. In their recent breakthrough published in Angewandte Chemie International Edition, Y. Liu and colleagues presented a new optimized hydrothermal synthesis approach that led to the synthesis of a Sn3O4 polymorph with a previously unreported orthorhombic crystal structure. The research was performed in the Mitsubishi Materials Sustainability Innovation Collaborative Research Cluster with the support of the Tokyo Institute of Technology Open Innovation Platform.
The project leader, Prof. Miyauchi explains the driving force behind the study, "The aim of our study was two-fold. First was the synthesis of a new tin oxide polymorph and the second was applying it for a visible-light sensitive photocatalyst."
The team set up multiple thermal hydrothermal reactors with the same starting material for preparing Sn3O4. In the first series one set, they altered the degree of filling of the precursor solution by filling 20, 40, 60, and 80% of a 100 ml Teflon liner. For the second series, they kept the degree of filling constant at 20% and the Teflon liners were filled with ambient air, pure oxygen, and pure nitrogen respectively.
The team then carried out Rietveld analysis, X-ray spectroscopy, and first-principles calculations on the products formed. The analysis showed the new Sn3O4 polymorph has the chemical formula of Sn(II)2Sn(IV)O4. Its X-ray diffraction pattern has never been reported and is assigned to an orthorhombic crystal phase based on empirical and computational analyses. The comparative studies for tuning of gas composition and degree of filling showed that the orthorhombic polymorph was only formed when the degree of filling was high or when the gas introduced was inert and has less oxygen. The team hence suggested that paying attention to the oxygen source could be the key to more precise hydrothermal synthesis.
The novel orthorhombic Sn3O4 polymorph reported in this study has a smaller bandgap than a conventional monoclinic Sn3O4, indicating a higher efficiency of absorbing visible light. Furthermore, the conduction band of the orthorhombic polymorph is enough high to drive CO2 reduction reaction.
Hydrothermal method is a widely used method of materials synthesis. This study finds that the often-neglected parameters in hydrothermal synthesis drastically affect the crystal structure. This finding is informative for the discovery of numerous new oxide materials.
More information: Yang-Shin Liu et al, Synthesis and Characterization of the Orthorhombic Sn3O4 Polymorph, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202300640
Journal information: Angewandte Chemie International Edition
Provided by Tokyo Institute of Technology