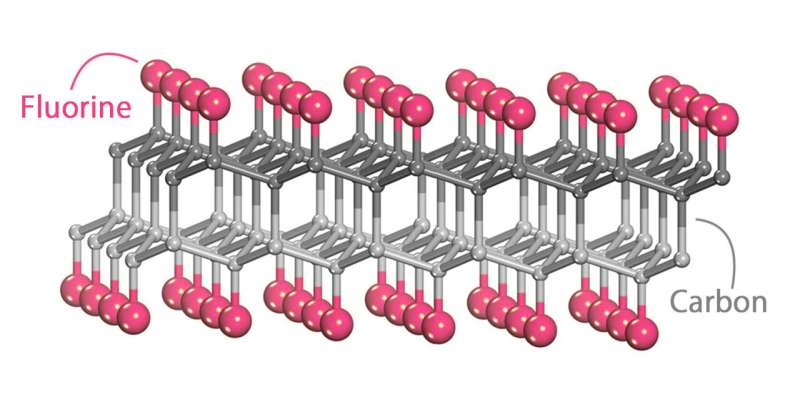
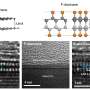
Diamane, an ultrathin diamond film comprised by carbon atoms—the gray spheres—which are coaxed into that arrangement by fluorine atoms—the cherry-colored spheres. Without fluorine, the carbon atoms would assume a honeycomb formation as two flat layers of graphene one on top of the other. Credit: Skoltech
Researchers from Skoltech, UiT the Arctic University of Norway, the Institute of Solid State Chemistry and Mechanochemistry SB RAS and their colleagues have theoretically investigated the properties of ultrathin diamond films and determined which of them hold the most promise for field-emission displays. These are an alternative flat-panel technology that used to be developed in parallel with the now-dominant LCDs and could one day make a comeback. The potential advantages are low power consumption, a wide viewing angle, and very fast response times, or how long it takes the display to change color. The study was published in ACS Applied Materials & Interfaces.
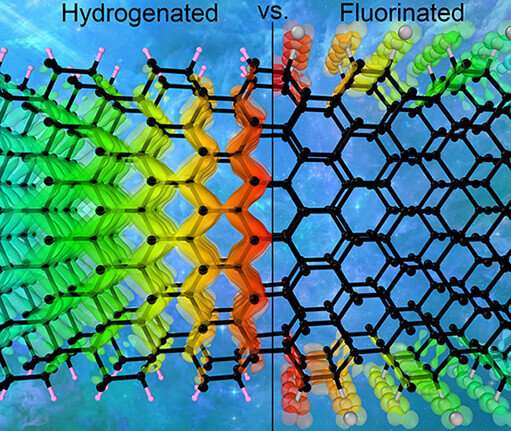
Diamane is an ultrathin diamond film created by stacking two or more layers of graphene on top of each other and attaching fluorine, hydrogen, or certain other atoms to the two outer surfaces. This warps the carbon layers and causes them to fuse into a diamond arrangement. The resulting material might just have the electronic properties that it takes to make field-emission displays for computers, cell phones, TVs, etc. But these properties depend on lots of variables and are hard to calculate.
Assistant Professor Alexander Kvashnin of Skoltech Energy Transition, who wrote a dissertation on diamane properties and co-authored the research reported in this story, said, "In this new study, we explore diamanes of different kinds, considering how various factors affect their electronic properties and hence their usability in field-emission displays. We analyzed 60 distinct diamane structures. You get this number by multiplying three variables: First, we considered thicknesses between one and six layers of carbon. Second, we used either fluorine or hydrogen atoms to induce the phase transition to diamond film. Third, there are five possible mutual orientations of graphene layers relative to each other, which you get by shifting the layers every which way."
Credit: ACS Applied Materials & Interfaces (2023). DOI: 10.1021/acsami.3c01536
A key characteristic determined by the researchers for each of the 60 diamane configurations is the amount of energy it takes to knock out an electron from the diamond film surface. This is a crucial parameter for field-emission displays, which rely on such electron emission to light up pixels making up the image on the screen. The less energy is expended for this, the better, but how much you actually need depends on the material's so-called band gap: which energy states are available to electrons in the solid and which are not. The authors of the study investigated that property of diamanes, too. Ultimately, the most suitable diamane for field-emission displays was defined to be the six-layer hydrogenated film with a (2̅110) surface.
Senior Research Scientist Christian Tantardini of the Institute of Solid State Chemistry and Mechanochemistry SB RAS and the Arctic University of Norway, had earned his Ph.D. at Skoltech and is the lead author of the new study. He added, "Besides the electron properties, we determined the surface dipole moments using a semiquantitative approach based on the electronegativity scale developed by myself and Professor Artem R. Oganov at Skoltech. This approach makes it possible to estimate the surface reactivity of the new two-dimensional materials without the highly complex and time-consuming first-principles calculations."
Surface dipole moments affect diamane electronic properties, including electron emission, and this information is therefore also valuable for designing field-emission displays and searching for alternative materials to be used in them.
More information: Christian Tantardini et al, Electronic Properties of Functionalized Diamanes for Field-Emission Displays, ACS Applied Materials & Interfaces (2023). DOI: 10.1021/acsami.3c01536
Journal information: ACS Applied Materials and Interfaces
Provided by Skolkovo Institute of Science and Technology